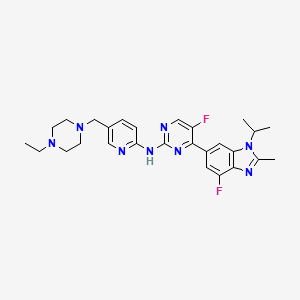

1. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzimidazol-5-yl)pyrimidin-2-yl)amine
2. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)pyrimidin-2-yl)amine
3. Abemaciclib Mesylate
4. Ly-2835219
5. Ly2385219
6. Ly2835210
7. Ly2835219
8. Verzenio
1. 1231929-97-7
2. Ly2835219
3. Verzenio
4. Ly2835219 Free Base
5. Ly-2835219
6. Unii-60uab198hk
7. N-(5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1h-benzo[d]imidazol-6-yl)pyrimidin-2-amine
8. N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine
9. Abemaciclib (ly2835219)
10. 60uab198hk
11. Ly 2835219
12. Hy-16297a
13. Cs-1230
14. 2-pyrimidinamine, N-(5-((4-ethyl-1-piperazinyl)methyl)-2-pyridinyl)-5-fluoro-4-(4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl)
15. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-1,3-benzodiazol-5-yl)pyrimidin-2-amine
16. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]pyrimidin-2-amine
17. Ly2835219 (free Base)
18. Abemaciclib [usan:inn]
19. Abemaciclib,ly2835219
20. Verzenios
21. Rimidin-2-amine
22. Verzenio (tn)
23. 6zv
24. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]py Rimidin-2-amine
25. Cdk4/6 Dual Inhibitor
26. Ly2835210
27. Abemaciclib [mi]
28. Abemaciclib [inn]
29. Abemaciclib [jan]
30. Abemaciclib (jan/usan)
31. Abemaciclib [usan]
32. Abemaciclib [who-dd]
33. Gtpl7382
34. Schembl2487229
35. Chembl3301610
36. Abemaciclib [orange Book]
37. Dtxsid20673119
38. Ex-a521
39. Ly 2835219 (free Base)
40. Hms3673i05
41. Bcp13079
42. Ex-a1588
43. Bdbm50110183
44. Mfcd22665744
45. Nsc768073
46. Nsc783671
47. S5716
48. Zinc72318121
49. 1231929-97-7, Verzenio,
50. Akos025404907
51. Ly2835219 Free Base (abemaciclib)
52. Ccg-269750
53. Db12001
54. Nsc-768073
55. Nsc-783671
56. Sb16476
57. Ncgc00351599-02
58. Ncgc00351599-06
59. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzimidazol-5-yl)pyrimidin-2-yl)amine
60. 5-(4-ethylpiperazin-1-ylmethyl)pyridin-2-yl)-(5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)pyrimidin-2-yl)amine
61. Ac-30666
62. As-10230
63. Da-33422
64. Ly2835219 Ms Salt, Abemaciclib Ms Salt
65. Ft-0700134
66. Ly 2835210
67. A12989
68. D10688
69. J-690083
70. Q23901483
71. [5-(4-ethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3h-benzoimidazol-5-yl)-pyrimidin-2-yl]-amine
72. 2-pyrimidinamine, N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl]-
73. 2-pyrimidinamine,n-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1h-benzimidazol-6-yl]-
74. N-{5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}-5-fluoro-4-[4-fluoro-2-methyl-1-(propan-2-yl)-1h-benzimidazol-6-yl]py
| Molecular Weight | 506.6 g/mol |
|---|---|
| Molecular Formula | C27H32F2N8 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 506.27179938 g/mol |
| Monoisotopic Mass | 506.27179938 g/mol |
| Topological Polar Surface Area | 75 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 723 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | VERZENIO |
| Active Ingredient | ABEMACICLIB |
| Company | ELI LILLY AND CO (Application Number: N208716. Patent: 7855211) |
* Indicated in combination with fulvestrant for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer with disease progression following endocrine therapy. * Inidicated as monotherapy for the treatment of adult patients with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting.
Early Breast Cancer
Verzenios in combination with endocrine therapy is indicated for the adjuvant treatment of adult patients with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative, node positive early breast cancer at high risk of recurrence (see section 5. 1).
In pre or perimenopausal women, aromatase inhibitor endocrine therapy should be combined with a luteinising hormone-releasing hormone (LHRH) agonist.
Advanced or Metastatic Breast Cancer
Verzenios is indicated for the treatment of women with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy.
In pre- or perimenopausal women, the endocrine therapy should be combined with a LHRH agonist.
Treatment of Ewing sarcoma
Treatment of breast cancer
Treatment of high-grade glioma, Treatment of neuroblastoma
In combination with fulvestrant, the progression-free survival for patients with HR-positive, HER2-negative breast cancer was 16.4 months compared to 9.3 months for patients taking a placebo with fulvestrant. As a monotherapy, 19.7% of patients taking abemaciclib achieved complete or partial shrinkage of their tumors for a median 8.6 months after treatment. Abemaciclib induces cell cycle arrest and exerts an antitumor activity in human tumor xenograft models. In patient investigations and a healthy volunteer study, abemaciclib is not shown to induce any clinically significant changes in the QTc interval.
L01EF03
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EF - Cyclin-dependent kinase (cdk) inhibitors
L01EF03 - Abemaciclib
Absorption
The plasma concentration of the drug increases in a dose-proportional manner. Following a single oral dose administration of 200 mg abemaciclib, the mean peak plasma concentration (Cmax) of 158 ng/mL is reached after 6 hours. The median time to reach maximum plasma concentration (Tmax) ranges from 4-6 hours following an oral administration of abemaciclib over a range of 50275 mg, but may range up to 24 hours. The absolute bioavailability of the drug is reported to be 45%.
Route of Elimination
Following a single oral dose of 150mg radiolabeled abemaciclib, approximately 81% of the total dose was recovered in feces while 3% of the dose was detected in urine. The majority of the drug is exceted as metabolites.
Volume of Distribution
The geometric mean systemic volume of distribution is approximately 690.3 L (49% CV).
Clearance
The geometric mean hepatic clearance (CL) of abemaciclib in patients was 26.0 L/h (51% CV).
Abemaciclib mainly undergoes hepatic metabolism mediated by CYP3A4. The major metabolite formed is N-desethylabemaciclib (M2), while other metabolites hydroxyabemaciclib (M20), hydroxy-N-desethylabemaciclib (M18), and an oxidative metabolite (M1) are also formed. M2, M18, and M20 are equipotent to abemaciclib and their AUCs accounted for 25%, 13%, and 26% of the total circulating analytes in plasma, respectively.
The mean plasma elimination half-life for abemaciclib in patients was 18.3 hours (72% CV).
Regulation of cell cycle is crucial in maintaining proper cell growth; dysregulated cell cycle signalling pathway is a key component in inducing hyperproliferation of cells and tumor formation in various cancers. G1 to S phase cell cycle progression, or transition through the G1 restriction point (R), is promoted by the retinoblastoma tumor suppressor protein (Rb)-mediated pathway. Activation of Rb-mediated pathway requires the interaction of Cyclin-dependent kinases (CDK) 4 and 6 with D-type cyclins, which drives the formation of active CDK4/CDK6 and subsequent phosphorylation of Rb. Rb is a tumor suppressant protein that inhibits proliferation through binding to and suppressing the activity of the E2F family of transcription factors. However, phosphorylation of Rb relieves suppression of E2F to allow expression of genes required for passage through the restriction point. This leads to increased expression of downstream signalling molecules and activity of protein kinases that promote the cell cycle progression and initiation of DNA replication. Phosphorylation of Rb and other proteins by CDK4/6 additionally leads to transcription of genes involved in cell cycle-independent activities including signal transduction, DNA repair transcriptional control, and mRNA processing. Abemaciclib selectively inhibits CDK4 and CDK6 with low nanomolar potency, inhibits Rb phosphorylation resulting in a G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells. Unlike other CDK inhibitors such as [DB09073] and [DB11730], abemaciclib exhibits greater selectivity for CDK4 compared to CDK6.