

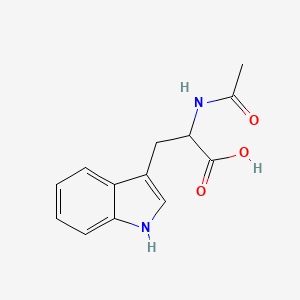

1. Acetyltryptophan
2. N-acetyl-d-tryptophan
3. N-acetyltryptophan
4. N-acetyltryptophanate Sodium
1. 87-32-1
2. Ac-dl-trp-oh
3. N-acetyltryptophan
4. Dl-acetyltryptophan
5. Acetyltryptophan
6. 2-acetamido-3-(1h-indol-3-yl)propanoic Acid
7. Tryptophan, N-acetyl-
8. Dl-n-acetyltryptophan
9. N-acetyl-dl-tryptophane
10. Dl-tryptophan, N-acetyl-
11. Nsc49124
12. Mfcd00005644
13. Nsc 49124
14. Tryptophan, N-acetyl-, Dl-
15. Chebi:70976
16. 4460nbv53f
17. N-acetyl-trp-oh
18. Nsc-49124
19. N-acetyl Tryptophan
20. N-acetyltryptophan #
21. N-acetyl-dl-tryptophen
22. Nalpha-acetyl-dl-tryptophan
23. N-alpha-acetyl-dl-tryptophan
24. Unii-4460nbv53f
25. Mfcd00065976
26. Nsc-90726
27. N-acetyltryptophane
28. Einecs 201-739-3
29. Acetyl-dl-tryptophan
30. N-a-acetyltryptophan
31. N-acetyl Dl-tryptophan
32. N-acetyl-d,l-tryptophan
33. D,l-alpha-acetylamino-3-indolepropionic Acid
34. Cambridge Id 5117020
35. N-
36. A-acetyl-dl-tryptophan
37. Nciopen2_005595
38. Oprea1_817403
39. Schembl57140
40. Acetyltryptophan, Dl-
41. Cbdive_014228
42. 2-(acetylamino)-3-(1h-indol-3-yl)propanoic Acid
43. Mls000686793
44. Chembl1905494
45. Acon1_001308
46. Bdbm91686
47. Dzthigrzjzprdv-uhfffaoysa-
48. Acetyltryptophan [who-dd]
49. Dtxsid40861672
50. Hms2271l03
51. Hms3372o11
52. Acetyltryptophan, Dl- [ii]
53. Act05774
54. Nsc90726
55. Bbl000688
56. Ccg-41706
57. Stk367673
58. Akos000120599
59. Akos016040255
60. Am82276
61. Cs-w012698
62. Hy-w011982
63. N-acetyltryptophan [ep Monograph]
64. Ac-19242
65. Ac-27041
66. Nci60_004180
67. Smr000339886
68. Sy036184
69. Sy036799
70. Vs-00668
71. Db-041633
72. Db-045994
73. Db-056991
74. A0120
75. Dl-.alpha.-acetamidoindole-3-propionic Acid
76. Ft-0629814
77. Ft-0633493
78. Ft-0634178
79. Dl-.alpha.-acetylamino-3-indolepropionic Acid
80. 2-acetamido-3-(1h-indol-3-yl)-propionic Acid
81. A-1810
82. A13889
83. D,l-.alpha.-acetylamino-3-indolepropionic Acid
84. 2-acetylamino-3-(1h-indol-3-yl)propionic Acid
85. Ab00637124-07
86. 005a644
87. A804795
88. Sr-01000597217
89. J-300264
90. Sr-01000597217-1
91. Sr-01000597217-2
92. Brd-a18626878-001-01-6
93. Q27139225
94. Z85881713
95. 3ad26bd1-c587-4301-9682-da13678ce54f
96. Ncgc00180645-03!2-acetamido-3-(1h-indol-3-yl)propanoic Acid
97. N-acetyl-dl-tryptophan, Pharmagrade, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production.
98. N-acetyl-dl-tryptophan, Pharmagrade, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production., Ep
| Molecular Weight | 246.26 g/mol |
|---|---|
| Molecular Formula | C13H14N2O3 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 246.10044231 g/mol |
| Monoisotopic Mass | 246.10044231 g/mol |
| Topological Polar Surface Area | 82.2 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 332 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)