

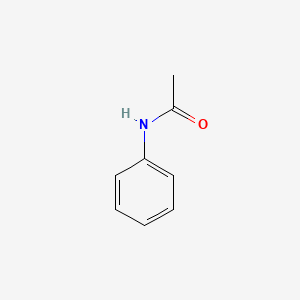

1. Acetanilid
1. N-phenylacetamide
2. 103-84-4
3. Antifebrin
4. Acetamidobenzene
5. Acetanilid
6. Acetanil
7. Acetylaniline
8. Acetamide, N-phenyl-
9. N-acetylaniline
10. Phenalgene
11. Acetic Acid Anilide
12. Acetylaminobenzene
13. Acetoanilide
14. Phenalgin
15. Aniline, N-acetyl-
16. N-acetylarylamine
17. Benzenamine, N-acetyl-
18. Usaf Ek-3
19. Nsc 7636
20. An [analgesic]
21. N-acetylaminobenzene
22. Acetanilidum
23. Antifebrinum
24. Ethananilide
25. Mfcd00008674
26. N-phenyl-acetamide
27. Acetanilide (antifebrin)
28. Nsc-7636
29. Sp86r356cc
30. Chebi:28884
31. Nsc7636
32. Acetamide, N-phenyl-, Homopolymer
33. N-phenylacetamide;n-phenylacetamide
34. Nsc-203231
35. Ncgc00091326-01
36. Dsstox_cid_2543
37. Dsstox_rid_76620
38. Dsstox_gsid_22543
39. N-phenylacetamide (acetanilide)
40. Caswell No. 003e
41. Acetanilide (ring-13c6)
42. N-phenylethanamide
43. Acetanilide [nf]
44. 137020-73-6
45. Cas-103-84-4
46. Smr001306799
47. Acetic Acid Amide, N-phenyl-
48. Ccris 4452
49. Hsdb 2665
50. Einecs 203-150-7
51. Unii-sp86r356cc
52. Ai3-01045
53. Phnhac
54. N-phenyl Acetamide
55. Acetanilide, 99%
56. Spectrum_000178
57. Acetanilide [mi]
58. Spectrum2_001434
59. Spectrum3_000935
60. Spectrum4_001034
61. Spectrum5_000989
62. Acetanilid [inci]
63. Acetanilide [hsdb]
64. Wln: 1vmr
65. Acetanilide (acetylaniline)
66. Acetanilide [vandf]
67. Acetanilidum [hpus]
68. Acetanilide-[ring-13c6]
69. Acetanilide [mart.]
70. Schembl24681
71. Acetanilide [usp-rs]
72. Acetanilide [who-dd]
73. Kbiogr_001587
74. Kbioss_000658
75. 55576-55-1
76. Mls002207284
77. Mls002415707
78. Divk1c_000073
79. Spectrum1501173
80. Spbio_001568
81. Chembl269644
82. Acetaminophen Related Compound D
83. Dtxsid2022543
84. Schembl14255226
85. Acetanilide, Lr, >=98.5%
86. Hms500d15
87. Kbio1_000073
88. Kbio2_000658
89. Kbio2_003226
90. Kbio2_005794
91. Kbio3_001970
92. Ninds_000073
93. Acetic Acid,amide,n-phenyl
94. Bcpp000440
95. Hms1921n07
96. Hms2092j11
97. Hms2234e18
98. Hms3374l04
99. Hms3651d07
100. Pharmakon1600-01501173
101. Zinc142824
102. Bcp02363
103. Tox21_111113
104. Tox21_200925
105. Ccg-38984
106. Nsc757879
107. S2538
108. Stk046402
109. Akos000121114
110. Tox21_111113_1
111. Bcp9000226
112. Nsc-757879
113. Acetanilide, Nist(r) Srm(r) 141d
114. Idi1_000073
115. Acetanilide Melting Point Standard
116. Ncgc00091326-02
117. Ncgc00091326-03
118. Ncgc00091326-04
119. Ncgc00091326-06
120. Ncgc00258479-01
121. As-13389
122. Sbi-0051673.p002
123. B2071
124. Cs-0010112
125. Ft-0621730
126. Ft-0621731
127. Ft-0661046
128. Ft-0661047
129. Ft-0661154
130. Paracetamol Impurity D [ep Impurity]
131. Sw220057-1
132. C07565
133. D72485
134. Ab00052235_07
135. Ab00052235_08
136. Acetanilide, Purified By Sublimation, >=99.9%
137. Acetanilide, Puriss. P.a., >=99.5% (chn)
138. Acetaminophen Related Compound D [usp-rs]
139. Ah-034/32461060
140. Q421761
141. Sr-05000001777
142. Q-200578
143. Sr-05000001777-1
144. Acetaminophen Related Compound D [usp Impurity]
145. Brd-k11094367-001-04-4
146. F0808-0907
147. Acetanilide, Zone-refined, Purified By Sublimation, >=99.95%
148. Acetaminophen Related Compound D, United States Pharmacopeia (usp) Reference Standard
149. Acetanilide Melting Point Standard, United States Pharmacopeia (usp) Reference Standard
150. Acetanilide (acetaminophen Related Compound D), Pharmaceutical Secondary Standard; Certified Reference Material
151. Acetanilide Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 135.16 g/mol |
|---|---|
| Molecular Formula | C8H9NO |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 135.068413911 g/mol |
| Monoisotopic Mass | 135.068413911 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 116 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
IT IS PARTICULARLY EFFECTIVE AS AN ANALGESIC IN PAIN OF THE NEURALGIC TYPE...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1053
MEDICATION (VET): ANALGESIC, ANTIPYRETIC AGENT
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 10
.../ACETANILID IS/ ESPECIALLY DANGEROUS IN ANEMIA, AFTER HEMORRHAGE AND DURING MENSTRUATION.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 81
...ITS USE AS AN ANALGESIC /IS CONDEMNED/ BECAUSE SAFER & EQUALLY EFFECTIVE DRUGS ARE AVAILABLE.
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 262
...NECROSIS OF RENAL PAPILLAE & TERMINAL TUBULES HAS BEEN ASSOCIATED WITH EXCESSIVE DOSES OF DRUG MIXTURES CONTAINING ACETOPHENETIDIN WITH...ACETANILID...
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 81
...ACETANILID.../PRODUCES/ HEMOLYTIC ANEMIA IN INDIVIDUALS WITH GENETIC DEFICIENCY OF ERYTHROCYTIC GLUCOSE-6-PHOSPHATE DEHYDROGENASE. DARK SKINNED RACES HAVE HIGHER INCIDENCE THAN LIGHT SKINNED BUT DISEASE HAS NOT BEEN NOTED IN NORTH AMERICAN INDIANS OR ESKIMOS.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 232
For more Drug Warnings (Complete) data for ACETANILIDE (6 total), please visit the HSDB record page.
/BETWEEN 4 & 5/: 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG, BETWEEN 1 TEASPOONFUL & 1 OZ FOR A 70 KG PERSON (150 LB). 5= EXTREMELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-50 MG/KG, BETWEEN 7 DROPS & 1 TEASPOONFUL FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-139
IT IS READILY EXCRETED IN THE URINE AS SULFATE OR GLUCURONATE CONJUGATE OF THE PHENOL /N-ACETYL-P-AMINOPHENOL/.
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 1835
36% OF ACETANILIDE DISSOLVED IN 0.1 N HCL (PKA 0.3) WAS ABSORBED FROM RAT STOMACH IN ONE HOUR. /FROM TABLE/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 27
...GREATER THAN 99.9% OF ACETANILIDE REMAINS UN-IONIZED @ BODY PH, FACILITATING ABSORPTION FROM BLOOD TO CEREBROSPINAL FLUID. THE BLOOD-CEREBROSPINAL FLUID PERMEABILITY COEFFICIENT IS 0.039/MIN. /FROM TABLE/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 82
.../ACETANILID IS/ READILY ABSORBED FROM GASTROINTESTINAL TRACT.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 80
For more Absorption, Distribution and Excretion (Complete) data for ACETANILIDE (14 total), please visit the HSDB record page.
.../RESEARCHERS/ FOUND THAT STARVATION DECREASED RATE OF METABOLISM OF...ACETANILIDE. ...STARVATION REDUCED OXIDATIVE PATHWAYS OF MALE RATS...
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 529
STRAIN DIFFERENCES /WITHIN THE SAME SPECIES/ WERE...NOTED IN ACETANILIDE...METABOLISM.
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 530
HEPATIC MICROSOMES FROM RABBITS WITH OBSTRUCTIVE JAUNDICE SHOW IMPAIRED METABOLISM OF ACETANILIDE...
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 530
2 NEW URINARY METABOLITES OF ACETANILIDE IN RATS THAT HAVE BEEN IDENTIFIED AS N-ACETYL-S-(5-ACETAMIDO-2-HYDROXYPHENYL)CYSTEINE & N-ACETYL-S-(3-ACETAMIDOPHENYL)CYSTEINE MAY HAVE BEEN FORMED VIA AN INTERMEDIATE EPOXIDE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 182
For more Metabolism/Metabolites (Complete) data for ACETANILIDE (20 total), please visit the HSDB record page.
Acetanilide has known human metabolites that include N-Hydroxy-N-phenylacetamide and acetaminophen.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
T/2 OF ACETANILID MEASURED IN POPULATIONS OF YOUNG (AGED 20-35 YR) & ELDERLY (AGED OVER 65 YR) PEOPLE (TOTAL= 93). T/2 WERE SIGNIFICANTLY LONGER IN THE ELDERLY.
FARAH F ET AL; HEPATIC DRUG ACETYLATION AND OXIDATION: EFFECTS OF AGING IN MAN; BR MED J 2(JUL 16) 155 (1977)