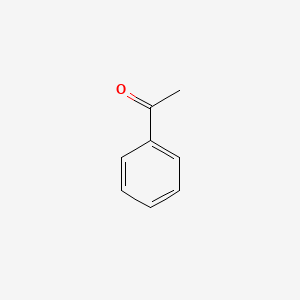

1. 98-86-2
2. 1-phenylethanone
3. Methyl Phenyl Ketone
4. Acetylbenzene
5. Phenyl Methyl Ketone
6. Ethanone, 1-phenyl-
7. Hypnone
8. Benzoyl Methide
9. Acetophenon
10. 1-phenylethan-1-one
11. Acetylbenzol
12. 1-phenyl-1-ethanone
13. Benzoylmethide
14. Benzene, Acetyl-
15. Phenylethanone
16. Acetofenon
17. Ketone, Methyl Phenyl
18. 1-phenyl-ethanone
19. Usaf Ek-496
20. Acetofenon [czech]
21. Rcra Waste Number U004
22. Hypnon
23. Methylphenylketone
24. Phenylmethylketone
25. Nsc 7635
26. Fema No. 2009
27. Dymex
28. Rcra Waste No. U004
29. Ai3-00575
30. Ketone, Methyl Phenyl-
31. Chembl274467
32. Rk493whv10
33. Chebi:27632
34. Nsc-7635
35. Dsstox_cid_1828
36. Dsstox_rid_76353
37. Dsstox_gsid_21828
38. Fema Number 2009
39. Cas-98-86-2
40. Ac0
41. (2,2,2,-2h3)acetophenone
42. Ccris 1341
43. Hsdb 969
44. Einecs 202-708-7
45. Unii-rk493whv10
46. Aceto Phenone
47. Aceto-phenone
48. Acetyl-benzen
49. Acetyl-benzene
50. Alpha-acetophenone
51. Ethanone,1-phenyl
52. Methyl-phenyl Ketone
53. Methyl Phenyl-ketone
54. Nchem.180-comp5
55. Acetophenone-[13c6]
56. 1-phenylethanone, 9ci
57. Acetophenone [ii]
58. Acetophenone [mi]
59. Schembl737
60. Acetophenone [fcc]
61. Bmse000286
62. Acetophenone [fhfi]
63. Acetophenone [hsdb]
64. Acetophenone [inci]
65. Ec 202-708-7
66. Wln: 1vr
67. Acetophenone, >=98%, Fg
68. Schembl8170205
69. 1-phenylethanone (acetophenone)
70. Dtxsid6021828
71. Schembl13341485
72. Acetophenone, Analytical Standard
73. Fema 2009
74. Nsc7635
75. Acetophenone, >=98.0% (gc)
76. Acetophenone, Natural, 98%, Fg
77. Zinc896628
78. Hy-y0989
79. Str00017
80. Tox21_202422
81. Tox21_300343
82. Acetophenone, Reagentplus(r), 99%
83. Bdbm50236986
84. C0117
85. Mfcd00008724
86. S5528
87. Akos000119011
88. Ccg-266074
89. Db04619
90. Ncgc00248000-01
91. Ncgc00248000-02
92. Ncgc00254370-01
93. Ncgc00259971-01
94. Acetophenone 100 Microg/ml In Cyclohexane
95. Db-044220
96. A0061
97. Cs-0015982
98. Ft-0628908
99. Ft-0636694
100. Ft-0637564
101. Ft-0641367
102. Ft-0661057
103. Acetophenone, Puriss. P.a., >=99.0% (gc)
104. C07113
105. D71016
106. A854783
107. Q375112
108. J-519533
109. Z57127548
110. Acetophenone, Tracecert(r), Certified Reference Material
| Molecular Weight | 120.15 g/mol |
|---|---|
| Molecular Formula | C8H8O |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 120.057514874 g/mol |
| Monoisotopic Mass | 120.057514874 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 101 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
... In rats acetophenone appears to be precursor of not only mandelic acid and benzoylformic acid but benzoic acid as well. ...
PMID:1274370 Sullivan HR et al; Xenobiotica 6 (1): 49-54 (1976)
The reductive cleavage of hydroperoxides by purified p450 in a reconstituted system containing reduced nicotinamide adenine dinucleotide phosphate was studied. ... With cumyl hydroperoxide, acetophenone was produced, but not cumyl alcohol, indicating that a rearrangement had taken place, probably involving radical intermediates, with the formation of an additional 1-carbon product.
Vaz ADN, Coon MJ; Proceedings of the Nat Acad of Sci 84 (5): 1172-6 (1987)
Early studies identified 1-phenylethanol, benzoic acid, and mandelic acid as urinary metabolites of acetophenone in rabbits and dogs. /It was/ found that rabbits administered acetophenone by gavage excreted 47% of the dose as glucuronide conjugates of 1-phenylethanol and about 20% as hippuric acid. /Another study/... reported that 1-phenylethanol and its glucuronide conjugate constituted only about 4% of the dose for rabbits treated by the ip route. ... m-Hydroxyacetophenone, p-hydroxyacetophenone, and w-hydroxyacetophenone as minor urinary metabolites of acetophenone (<1% of the dose) in rabbits.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 297
...10% of a 100 mg/kg ip dose of radiolabeled acetophenone was excreted as /carbon dioxide/ after 4 hr and that the amount increased to 30% after 13 hr. ... Mendelic acid was present in the urine of rats treated ip with acetophenone and ... this metabolite most likely arose from w-hydroxyacetophenone.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 297