
1. Acid, Hydrocyanic
2. Cyanide, Hydrogen
3. Hydrocyanic Acid
4. Zyklon B
1. Hydrocyanic Acid
2. Formonitrile
3. Prussic Acid
4. Cyanwasserstoff
5. Blausaeure
6. Formic Anammonide
7. Evercyn
8. 74-90-8
9. Zaclondiscoids
10. Cyclon
11. Cyclone B
12. Aero Liquid Hcn
13. Agent Ac
14. Cyaanwaterstof
15. Cyjanowodor
16. Blauwzuur
17. Acido Cianidrico
18. Acide Cyanhydrique
19. Hydrogen Cyanide [iso]
20. Rcra Waste Number P063
21. Blausaeure [german]
22. Carbon Hydride Nitride (chn)
23. Methanenitrile
24. Hcn
25. Hydridonitridocarbon
26. [chn]
27. Un 1051
28. Hydrogen(nitridocarbonate)
29. Zyklon B
30. 2wtb3v159f
31. Chembl183419
32. Chebi:18407
33. Blausaeure (german)
34. Blauwzuur [dutch]
35. Cyjanowodor [polish]
36. Caswell No. 483
37. Cyaanwaterstof [dutch]
38. Cyanwasserstoff [german]
39. Acido Cianidrico [italian]
40. Acide Cyanhydrique [french]
41. Ac [cyanide]
42. Hsdb 165
43. Acide Cyanhydrique [iso-french]
44. Einecs 200-821-6
45. Na1613
46. Un1051
47. Un1613
48. Un1614
49. Un3294
50. Rcra Waste No. P063
51. Epa Pesticide Chemical Code 045801
52. Ai3-31100-x
53. Brn 1718793
54. Unii-2wtb3v159f
55. Zootic Acid
56. Nitrilomethane #
57. Prussic Acid, Anhydrous, Stabilized
58. Carbon Hydride Nitride
59. Hydrocyanic Acid, Anhydrous, Stabilized
60. Hydrogen Cyanide, Anhydrous, Stabilized
61. Prussic Acid, Unstabilized
62. Hydrocyanicum Acidum
63. Ec 200-821-6
64. 143334-20-7
65. Un 1613 (salt/mix)
66. Un 1614 (salt/mix)
67. Hydrogen Cyanide [mi]
68. Hydrocyanic Acid (prussic), Unstabilized [forbidden]
69. Hydrogen Cyanide [hsdb]
70. Dtxsid9024148
71. Hydrogen Cyanide [who-dd]
72. Hydrocyanicum Acidum [hpus]
73. Ac (chemical Warfare Agent)
74. Bdbm50152968
75. Hydrocyanic Acid Aqueous Solutions, With Not >20% Hydrocyanic Acid
76. Hydrocyanic Acid, Anhydrous, Stabilized, Absorbed In A Porous Inert Material
77. Hydrogen Cyanide, Anhydrous, Stabilized, Absorbed In A Porous Inert Material
78. Prussic Acid, Anhydrous, Stabilized, Absorbed In A Porous Inert Material
79. Na 1051
80. Hydrocyanic Acid (prussic), Unstabilized
81. Hydrogen Cyanide, Stabilized With <3% Water
82. C01326
83. Q3416481
84. Graphitic Carbon Nitride, 99%, Length: 1 - 10 Mum
85. Hydrocyanic Acid, Aqueous Solutions <5% Hydrogen Cyanide
86. Hydridonitridocarbonhydrogen(nitridocarbonate)methanenitrile
87. Hydrogen Cyanide, Stabilized With <3% Water [un1051] [poison]
88. Hydrogen Cyanide, Solution In Alcohol With Not >45% Hydrogen Cyanide
89. Hydrocyanic Acid, Aqueous Solutions <5% Hydrogen Cyanide [na1613] [poison]
90. Hydrogen Cyanide, Solution In Alcohol With Not >45% Hydrogen Cyanide [un3294] [poison]
91. Hydrogen Cyanide, Stabilized, With <3% Water And Absorbed In A Porous Inert Material
92. Hydrocyanic Acid, Aqueous Solutions Or Hydrogen Cyanide, Aqueous Solutions With Not > 20% Hydrogen Cyanide
93. Hydrocyanic Acid, Aqueous Solutions Or Hydrogen Cyanide, Aqueous Solutions With Not > 20% Hydrogen Cyanide [un1613] [poison]
94. Hydrogen Cyanide, Stabilized, With <3% Water And Absorbed In A Porous Inert Material [un1614] [poison]
| Molecular Weight | 27.025 g/mol |
|---|---|
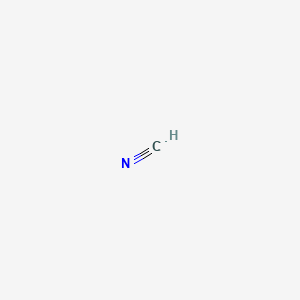
| Molecular Formula | CHN |
| XLogP3 | 0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 27.010899036 g/mol |
| Monoisotopic Mass | 27.010899036 g/mol |
| Topological Polar Surface Area | 23.8 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cyanide is the least toxic of the "lethal" chemical agents. The LCt50s of hydrogen cyanide (AC) and cyanogen chloride (CK) by inhalation have been estimated to be 2500-5000 mg x min/cu m for AC and about 11,000 mg x min/cu m for CK. LD50s for hydrogen cyanide have been estimated to be 1.1 mg/kg for IV administration and 100 mg/kg after skin exposure. ... Cyanide is unique among military chemical agents because it is detoxified at a rate that is of practical importance, about 17 ug/kg x min. As a result the LCt50 is greater for a long exposure (e.g., 60 minutes) than for a short exposure (e.g., 2 minutes).
U.S. Army Research Institute of Chemical Defense, Chemical Casualty Care Division; Medical Management of Chemical Casualties Handbook, 3rd Ed. Aberdeen Proving Ground, MD (August 1999) Available from, as of January 5, 2018: https://www.brooksidepress.org/Products/OperationalMedicine/DATA/operationalmed/Manuals/RedHandbook/001TitlePage.htm
Cyanide is a potent oral poison producing symptoms in minutes and death in minutes to hours. One teaspoon of 29% liquid hydrogen cyanide has been fatal.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1477
Lethal adult dose of hydrogen cyanide is 50 mg.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1477
An average LD50 value for dermal exposure of 100 mg CN per kg as hydrogen cyanide was estimated for humans.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.66 (July 2006)
An average fatal concentration for humans was estimated as 546 ppm hydrogen cyanide after a 10-minute exposure.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide. p.27 (July 2006)
An average LD50 value for dermal exposure of 100 mg/kg body weight was estimated for humans. Concentrations of 7000 to 12,000 mg/cu m were estimated to be fatal after a 5-min exposure of workers with self-contained respirators without effective skin protection.
International Programme on Chemical Safety; Concise International Chemical Assessment Document 61: Hydrogen Cyanide and Cyanides: Human Health Aspects (2004) Available from, as of January 5, 2018: https://www.inchem.org/documents/cicads/cicads/cicad61.htm
Chemical Warfare Agents
Chemicals that are used to cause the disturbance, disease, or death of humans during WARFARE. (See all compounds classified as Chemical Warfare Agents.)
Cyanide as hydrogen cyanide is rapidly absorbed (within seconds) following inhalation exposure. Humans retained 58% of hydrogen cyanide in the lungs after inhaling the gas through normal breathing.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.75 (July 2006)
Once cyanide is absorbed, it is rapidly distributed by the blood throughout the body. Tissue levels of hydrogen cyanide were 0.75, 0.42, 0.41, 0.33, and 0.32 mg/100 g of tissue in the lung, heart, blood, kidney, and brain, respectively, in a man who died following inhalation exposure to hydrogen cyanide gas. ... In another case, tissue cyanide levels from a man who died from inhalation of hydrogen cyanide were reported as 0.5 mg per 100 mL of blood and 0.11, 0.07, and 0.03 mg/100 g in the kidney, brain, and liver, respectively. Urinary cyanide levels were reported as 0.2 mg/100 mL, and 0.03 mg/100 g were found in the gastric contents ... .
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.77-8 (July 2006)
Rats exposed /by inhalation/ to hydrogen cyanide gas at 356 or 1,180 ppm died within 10 and 5 minutes, respectively. Samples taken immediately after respiration stopped showed that the pattern of tissue distribution of cyanide did not vary with the concentration used. In averaging data for both dose groups, tissue concentrations, reported as ug/g wet weight (ww), were 4.4 in the lungs, 3.0 in the blood, 2.15 in the liver, 1.4 in the brain, and 0.68 in the spleen. Thus, the highest cyanide concentrations were observed in the lung.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.78 (July 2006)
Rabbits exposed /by inhalation/ to hydrogen cyanide at 2,714 ppm for 5 minutes had cyanide levels of 170/100 mL in blood and 48 ug/100 mL in plasma, and tissue levels (in units of ug/100 g) of 0 in the liver, 6 in the kidney, 50 in the brain, 62 in the heart, 54 in the lung, and 6 in the spleen. ... Six rabbits exposed dermally (area not reported) to 33.75 mg CN/kg as hydrogen cyanide had blood and serum cyanide levels of 310 and 144 ug/dL, respectively, and tissue levels (ug/100 g) of 26 in liver, 66 in kidney, 97 in brain, 110 in heart, 120 in lungs, and 21 in the spleen.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.78, 80 (July 2006)
For more Absorption, Distribution and Excretion (Complete) data for Hydrogen cyanide (27 total), please visit the HSDB record page.
Reports of ingestion of cyanides by humans and reports of occupational exposure ... indicate that cyanide is transformed into thiocyanate. ... Conversion of cyanide to thiocyanate is enhanced when cyanide poisoning is treated by intravenous administration of a sulfur donor ... . The sulfur donor must have a sulfane sulfur, a sulfur bonded to another sulfur (e.g., sodium thiosulfate). During conversion by rhodanese, a sulfur atom is transferred from the donor to the enzyme, forming a persulfide intermediate. The persulfide sulfur is then transferred from the enzyme to cyanide, yielding thiocyanate. Thiocyanate is then readily excreted in the urine as the major metabolite. Once thiocyanate is formed, it is not converted back to cyanide. /Cyanide/
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.80-2 (July 2006)
The metabolism of cyanide has been studied in animals. The proposed metabolic pathways ... are (1) the major pathway, conversion to thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase; (2) conversion to 2-aminothiazoline-4-carboxylic acid; (3) incorporation into a 1-carbon metabolic pool; or (4) combining with hydroxocobalamin to form cyanocobalamin (vitamin B12). Thiocyanate has been shown to account for 60 to 80% of an administered cyanide dose while 2-aminothiazoline-4-carboxylic acid accounts for about 15% of the dose. /Cyanide/
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.80 (July 2006)
Although cyanide can interact with substances such as methemoglobin in the bloodstream, the majority of cyanide metabolism occurs within the tissues. Cyanide is metabolized in mammalian systems by one major route and several minor routes. The major route of metabolism for hydrogen cyanide and cyanides is detoxification in the liver by the mitochondrial enzyme rhodanese, which catalyses the transfer of the sulfane sulfur of thiosulfate to the cyanide ion to form thiocyanate ... . About 80% of cyanide is detoxified by this route. The rate-limiting step is the amount of thiosulfate. While rhodanese is present in the mitochondria of all tissues, the species and tissue distributions of rhodanese are highly variable. In general, the highest concentrations of rhodanese are found in the liver, kidney, brain, and muscle, but the supply of thiosulfate is limited. Rhodanese is present in rat nasal mucosal tissues, particularly in the olfactory region, at a 7-fold higher concentration (on a per milligram of mitochondrial protein basis) than in the liver. Dogs have a lower overall activity of rhodanese than monkeys, rats, and rabbits. A number of other sulfur transferases can also metabolize cyanide, and albumin, which carries elemental sulfur in the body in the sulfane form, can assist in the catalysis of cyanide to thiocyanate as well. Cyanide and thiocyanate can also be metabolized by several minor routes, including the combination of cyanide with hydroxycobalamin (vitamin B12a) to yield cyanocobalamin (vitamin B12) and the non-enzymatic combination of cyanide with cystine, forming 2-iminothiazoline-4-carboxylic acid, which appears to be excreted without further change. /Cyanide/
International Programme on Chemical Safety; Concise International Chemical Assessment Document 61: Hydrogen Cyanide and Cyanides: Human Health Aspects (2004) Available from, as of January 5, 2018: https://www.inchem.org/documents/cicads/cicads/cicad61.htm
While absorbed cyanide is principally excreted as thiocyanate in the urine, traces of free hydrogen cyanide may also be excreted unchanged in the lungs, saliva, sweat, or urine, as carbon dioxide in expired air, or as beta-thiocyanoalanine in saliva and sweat. /Cyanide/
International Programme on Chemical Safety; Concise International Chemical Assessment Document 61: Hydrogen Cyanide and Cyanides: Human Health Aspects (2004) Available from, as of January 5, 2018: https://www.inchem.org/documents/cicads/cicads/cicad61.htm
For more Metabolism/Metabolites (Complete) data for Hydrogen cyanide (17 total), please visit the HSDB record page.
The time-course of cyanide in exhaled air was measured with an electrochemical detector in 10 volunteers during and after a 1 min x 10 ppm exposure to HCN. The experiment revealed an average half-life of 16s (range 10-24s) in breath.
PMID:18490114 Stamyr K et al; Toxicol Lett 179 (1): 59-62 (2008)
A plasma half-life of 20 minutes to 1 hour has been estimated for cyanides in humans after nonlethal exposures /Cyanides/
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.80 (July 2006)
Half-time values of the principal metabolite thiocyanate in humans have been reported as 4 hr, 2 days, and 2.7 days. In patients with renal insufficiency, a mean half-time of 9 days was reported.
International Programme on Chemical Safety; Concise International Chemical Assessment Document 61: Hydrogen Cyanide and Cyanides: Human Health Aspects (2004) Available from, as of January 5, 2018: https://www.inchem.org/documents/cicads/cicads/cicad61.htm
Half-life for the conversion of cyanide to thiocyanate from a non-lethal dose in man is between 20 min and 1 hr. /Cyanide/
Feldstein M, Klendshoj NC; J Lab Chin Med 44: 166-70 (1954) as cited in NIOSH; Criteria Document: Hydrogen Cyanide and Cyanide Salts p.45 (1976) DHEW Pub. NIOSH 77-108
Cyanide (as hydrogen cyanide), originating in vivo by dissociation of potassium cyanide, sodium cyanide, and other cyanogenic compounds or arising from catabolism of cyanogenic glycosides, exerts its acute toxic effects by complexing with the ferric iron atom in metalloenzymes, resulting in histotoxic anoxia through inhibition of cytochrome c oxidase, metalloenzymes that function as the terminal oxidase of the inner mitochondrial membrane respiratory chain. A two-step process has been proposed: cyanide as hydrogen cyanide first penetrates a protein crevice of cytochrome c oxidase and binds to the protein. Hydrogen cyanide then binds to the trivalent iron ion of the enzyme, forming a relatively stable (but reversible) coordination complex. One mole of hydrogen cyanide is bound to one mole of cytochrome c oxidase. As a result, the enzyme becomes unable to catalyze the reactions in which electrons would be transferred from reduced cytochrome to oxygen. Cellular oxygen utilization is thus impaired, with resultant reduction in or cessation of aerobic metabolism. Glucose catabolism then shifts from the aerobic pathway to anaerobic metabolism including the pentose phosphate pathway, resulting in increased blood glucose, pyruvic acid, lactic acid, and nicotinamide adenine dinucleotide (NADPH) levels, and a decrease in the adenosine triphosphate/adenosine diphosphate (ATP/ADP) ratio. /Some investigators/ suggest that it is the binding of cyanide to oxidized CuB, the copper ion that is part of the dioxygen binding-site that leads to the inhibition of cytochrome c oxidase. The inhibition of oxygen use by cells (termed histoxic hypoxia) causes oxygen tensions to rise in peripheral tissues. This results in a decrease in the unloading gradient for oxyhemoglobin; thus, oxyhemoglobin is carried in the venous blood. Inhibition of oxygen utilization is thought to occur rapidly after cyanide exposure. ... In addition to binding to cytochrome c oxidase, cyanide also binds to catalase, peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, and succinic dehydrogenase. These reactions may also contribute to the classic signs of cyanide toxicity. ...
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Cyanide p.90-1(July 2006)
The mitochondrial electron transport system may also be seriously disturbed by cyanide through the inhibition of succinate dehydrogenase, which is a nonheme flavin-containing iron-sulfur protein which passes electrons to the cytochrome system. The peculiarity of this enzyme is its sulfur linkages which are of the persulfide or sulfane type, favoring a "labile sulfur" condition that is essential for activity. Cyanide is a strong thiophile reacting with the "labile" sulfur thus breaking the persulfide bond.
Nat'l Research Council Canada; The Effect of Cyanides on Aquatic Organisms with Emphasis Upon Fresh Water Fishes p.59 (1982) NRCC No.19246