

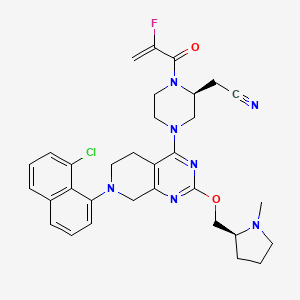

1. 2-((s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((s)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile
2. 2-piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2s)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
3. Mrtx-849
4. Mrtx849
1. Mrtx849
2. 2326521-71-3
3. Mrtx-849
4. Adagrasib [usan]
5. Kras G12c Inhibitor Mrtx849
6. 8eoo6hqf8y
7. 2-((s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((s)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile
8. 2-[(2s)-4-[7-(8-chloronaphthalen-1-yl)-2-[[(2s)-1-methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5h-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
9. 2-piperazineacetonitrile, 4-[7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-[[(2s)-1-methyl-2-pyrrolidinyl]methoxy]pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
10. 2-piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2s)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
11. Adagrasib [inn]
12. Unii-8eoo6hqf8y
13. Adagrasib [who-dd]
14. Chembl4594350
15. Schembl20974691
16. Gtpl10888
17. Dtxsid801336759
18. Bcp31538
19. Ex-a3258
20. Mrtx-849; Mrtx 849
21. Bdbm50539763
22. Mfcd32263433
23. Nsc831453
24. S8884
25. Who 11519
26. Akos037648997
27. At23561
28. Nsc-831453
29. Compound 20 [pmid: 32250617]
30. Ac-35659
31. Bm177692
32. Bs-16211
33. Hy-130149
34. Cs-0105265
35. A936721
36. ((2s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((2s)-1- Methylpyrrolidin-2-yl)methoxy)-5,6,7,8- Tetrahydropyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoroprop2-enoyl)piperazin-2-yl)acetonitrile
37. [(2s)-4-[7-(8-chloro-1-naphthyl)-2-{[(2s)-1-methyl-2-pyrrolidinyl]methoxy}-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroacryloyl)-2-piperazinyl]acetonitrile
| Molecular Weight | 604.1 g/mol |
|---|---|
| Molecular Formula | C32H35ClFN7O2 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 603.2524792 g/mol |
| Monoisotopic Mass | 603.2524792 g/mol |
| Topological Polar Surface Area | 88.8 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1060 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MRTX849 is an experimental KRAS inhibitor being investigated for the treatment of KRAS G12C mutant lung and colon adenocarcinomas.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway. GTP is hydrolyzed to GDP, and KRAS is inactivated. KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. MRTX849 inhibits KRAS in these types of cancers. This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors.