
1. 271, Cd
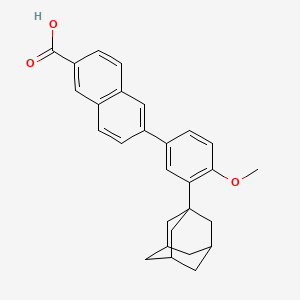
2. 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic Acid
3. Adaferin
4. Cd 271
5. Cd-271
6. Cd271
7. Differin
8. Differine
1. 106685-40-9
2. Differin
3. Adapaleno
4. 6-(3-(adamantan-1-yl)-4-methoxyphenyl)-2-naphthoic Acid
5. Adapalenum
6. Adapalenum [inn-latin]
7. Adapaleno [inn-spanish]
8. Cd 271
9. Cd-271
10. 6-[3-(1-adamantyl)-4-methoxyphenyl]naphthalene-2-carboxylic Acid
11. 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic Acid
12. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic Acid
13. Mfcd03106112
14. 6-[3-(1-adamantyl)-4-methoxy-phenyl]naphthalene-2-carboxylic Acid
15. Chembl1265
16. 6-(4-methoxy-3-tricyclo[3.3.1.13,7]dec-1-ylphenyl)-2-naphthalenecarboxylic Acid
17. Adaferin
18. Chebi:31174
19. 1l4806j2qf
20. 6-(3-adamantan-1-yl-4-methoxyphenyl)naphthalene-2-carboxylic Acid
21. Ncgc00164617-01
22. Adapalen
23. Differine
24. 6-[3-(adamantan-1-yl)-4-methoxyphenyl]naphthalene-2-carboxylic Acid
25. 2-naphthalenecarboxylic Acid, 6-(4-methoxy-3-tricyclo(3.3.1.1(sup 3,7))dec-1-ylphenyl)-
26. Dsstox_cid_26481
27. Dsstox_rid_81652
28. Dsstox_gsid_46481
29. Differin Gel
30. Cd271
31. Differin (tn)
32. Cas-106685-40-9
33. Differin Gel 0.1%
34. Adapalene [usan:inn:ban]
35. Unii-1l4806j2qf
36. Adapalene- Bio-x
37. Ks-1196
38. Adapalene [inn]
39. Adapalene [jan]
40. Adapalene [mi]
41. Adapalene [usan]
42. Adapalene [vandf]
43. Adapalene [mart.]
44. Adapalene [usp-rs]
45. Adapalene [who-dd]
46. Schembl2747
47. Adapalene (jan/usp/inn)
48. Mls000759463
49. Mls006010036
50. Bidd:gt0264
51. Idp-126 Component Adapalene
52. Gtpl5429
53. Adapalene [orange Book]
54. Adapalene [ep Monograph]
55. Adapalene, >=98% (hplc)
56. Chembl4303650
57. Dtxsid5046481
58. Epiduo Component Adapalene
59. Adapalene [usp Monograph]
60. Hms3264f15
61. Hms3654f11
62. Hms3715h16
63. Bcp02081
64. Hy-b0091
65. Zinc3784182
66. Adapalene Component Of Epiduo
67. Tox21_112236
68. Bdbm50048280
69. S1276
70. Stl453114
71. Akos005145841
72. Akos015895391
73. Tox21_112236_1
74. Ab13763
75. Ac-1974
76. Bcp9000231
77. Ccg-213060
78. Ccg-221237
79. Cs-1789
80. Db00210
81. Ncgc00164617-02
82. Ncgc00164617-04
83. Ncgc00164617-05
84. Ba164138
85. Smr000466349
86. Smr002529673
87. Sy009767
88. A2549
89. Ft-0631040
90. Sw219282-1
91. D01112
92. Ab01274764-01
93. Ab01274764-02
94. Ab01274764_03
95. Ab01274764_04
96. 685a409
97. A801483
98. Q352348
99. Sr-01000942194
100. Sr-01000942194-2
101. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoicacid
102. Brd-k33127281-001-01-5
103. F2173-0588
104. 6-(3-(adamantan-1-yl)-4-methoxyphenyl)-2-naphthoicacid
105. Adapalene 100 Microg/ml In Acetonitrile:dimethylsulfoxide
106. Adapalene, European Pharmacopoeia (ep) Reference Standard
107. Adapalene, United States Pharmacopeia (usp) Reference Standard
108. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthalene-carboxylic Acid
109. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthalenecarboxylic Acid
110. 6-(3-((3r,5r,7r)-adamantan-1-yl)-4-methoxyphenyl)-2-naphthoic Acid
111. Adapalene, Pharmaceutical Secondary Standard; Certified Reference Material
112. 6-[3-(1-adamantyl)-4-methoxy-phenyl]naphthalene-2-carboxylic Acid;adapalene
113. 6-[4-methoxy-3-(tricyclo[3.3.1.1~3,7~]dec-1-yl)phenyl]naphthalene-2-carboxylic Acid
114. 6-[4-methoxy-3-(tricyclo[3.3.1.13,7]dec-1-yl)phenyl]naphthalene-2-carboxylic Acid
115. Adapalene For Peak Identification, European Pharmacopoeia (ep) Reference Standard
116. 2-naphthalenecarboxylic Acid, 6-(4-methoxy-3-tricyclo(3.3.1.(sup 13,7))dec-1-ylphenyl)-
| Molecular Weight | 412.5 g/mol |
|---|---|
| Molecular Formula | C28H28O3 |
| XLogP3 | 7.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 412.20384475 g/mol |
| Monoisotopic Mass | 412.20384475 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Adapalene |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | Adapalene Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of Adapalene Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer homopolymer type C, disodium edetate, methylparaben, poloxamer 182,... |
| Active Ingredient | Adapalene |
| Dosage Form | Gel; Cream |
| Route | topical; Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Glenmark Generics; Fougera Pharms; Actavis Mid Atlantic; Pliva Hrvatska Doo; Tolmar |
| 2 of 4 | |
|---|---|
| Drug Name | Differin |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | DIFFERIN Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of DIFFERIN Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glyc... |
| Active Ingredient | Adapalene |
| Dosage Form | Lotion; Gel; Cream |
| Route | Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Adapalene |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | Adapalene Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of Adapalene Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer homopolymer type C, disodium edetate, methylparaben, poloxamer 182,... |
| Active Ingredient | Adapalene |
| Dosage Form | Gel; Cream |
| Route | topical; Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Glenmark Generics; Fougera Pharms; Actavis Mid Atlantic; Pliva Hrvatska Doo; Tolmar |
| 4 of 4 | |
|---|---|
| Drug Name | Differin |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | DIFFERIN Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of DIFFERIN Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glyc... |
| Active Ingredient | Adapalene |
| Dosage Form | Lotion; Gel; Cream |
| Route | Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Prescription |
| Company | Galderma Labs |
Adapalene is indicated for the topical treatment of acne vulgaris in patients aged 12 and over.
FDA Label
Adapalene is anticomedogenic, preventing the formation of new comedones and inflammatory lesions, and also acts to reduce inflammation by modulating the innate immune response. Like other retinoid compounds, adapalene is chemically stable but photosensitive; use with sunscreen is recommended. Minor skin irritations, including erythema, scaling, dryness, and stinging/burning, have been reported.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D10AD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AD - Retinoids for topical use in acne
D10AD03 - Adapalene
Absorption
Adapalene is applied topically and absorbed through the skin. In one clinical study treating patients once per day with 2g of 0.3% gel applied to 2 mg/cm2 of skin, 15 patients had detectable blood plasma adapalene levels (0.1 ng/ml) resulting in a mean Cmax of 0.553 0.466 ng/ml and a mean AUC of 8.37 8.46 ng\*h/ml on day 10.
Route of Elimination
Adapalene is primarily excreted by the biliary route at about 30 ng/g of the topically applied amount. Approximately 75% of the drug remains unchanged.
Clearance
Adapalene is rapidly cleared from blood plasma, typically undetectable after 72 hours following topical application.
Extensive information regarding adapalene metabolism in humans is unavailable, although it is known to accumulate in the liver and GI-tract. In human, mouse, rat, rabbit, and dog cultured hepatocytes, metabolism appears to affect the methoxybenzene moiety but remains incompletely characterized. The major products of metabolism are glucuronides. Approximately 25% of the drug is metabolized; the rest is excreted as parent drug.
In one clinical study, after ten days of treatment with 2g of 0.3% cream or gel, the terminal half-life was between 7 and 51 hours, with a mean of 17.2 10.2.
Adapalene is used for the treatment/maintenance of mild-to-severe acne (acne vulgaris). Acne is a multifactorial condition, and evidence exists to support multiple mechanisms of action for adapalene. Adapalene binds to retinoic acid receptor (RAR)-beta and RAR-gamma; this complex subsequently binds to one of three retinoid X receptors (RXRs), which as a complex is capable of binding DNA to modulate transcriptional activity. Although the full extent of transcriptional modulation is not described, retinoid activation is generally known to affect cellular proliferation and differentiation, and adapalene has been shown to inhibit HeLa cell proliferation and human keratinocyte differentiation. These effects primarily account for adapalene's comedolytic and anticomedogenic properties. In addition, adapalene modulates the immune response by down-regulating toll-like receptor 2 (TLR-2) expression and inhibiting the transcription factor activator protein 1 (AP-1). TLR-2 recognizes _Cutibacterium acnes_ (formerly _Propionibacterium acnes_), the bacterium primarily associated with acne. TLR-2 activation causes nuclear translocation of AP-1 and downstream pro-inflammatory gene regulation. Therefore, adapalene has a general anti-inflammatory effect, which reduces inflammation-mediated acne symptoms. When used with benzoyl peroxide, which possesses free radical-mediated bactericidal effects, the combination acts synergistically to reduced comedones and inflammatory lesions.