


1. 56287-74-2
2. Arofuto
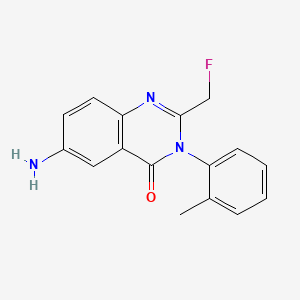
3. 4(3h)-quinazolinone, 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)-
4. Airomate
5. Hq-495
6. Co4u2c8orz
7. 6-amino-2-(fluoromethyl)-3-(o-tolyl)quinazolin-4(3h)-one
8. 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)quinazolin-4-one
9. 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)-4(3h)-quinazolinone
10. 6-amino-2-(fluoromethyl)-3-o-tolyl-4(3h)-quinazolinone
11. Afloqualon
12. 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)-quinazolin-4-one
13. 6-amino-2-(fluoromethyl)-3-o-tolylquinazolin-4(3h)-one
14. Afloqualona
15. Afloqualonum
16. Afloqualone [inn:jan]
17. Afloqualonum [inn-latin]
18. Afloqualona [inn-spanish]
19. Unii-co4u2c8orz
20. Hq 495
21. Brn 0819769
22. Airomate (tn)
23. 6-amino-2-(fluoromethyl)-3-o-tolyl-4(3h)-chinazolinon
24. Afloqualone [mi]
25. 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)quinazolin-4(3h)-one
26. Afloqualone [inn]
27. Afloqualone [jan]
28. 6-amino-3,4-dihydro-2-fluoromethyl-3-(2-methylphenyl)-4-chinazolinon
29. Dsstox_cid_2562
30. Afloqualone (jp17/inn)
31. Afloqualone [mart.]
32. Dsstox_rid_76631
33. Dsstox_gsid_22562
34. Afloqualone [who-dd]
35. Mls006010086
36. Schembl400184
37. Chembl2105918
38. Dtxsid5022562
39. Chebi:31177
40. 6-amino-2-(fluoromethyl)-3-(2-methylphenyl)-4-quinazolinone
41. Hms3885g19
42. Bcp13264
43. Hy-b1833
44. Tox21_112447
45. Mfcd00867693
46. S3663
47. Zinc13831145
48. Akos015842375
49. Ac-1608
50. Ccg-267278
51. Cs-5009
52. Ncgc00167443-02
53. As-11726
54. Smr004701231
55. Cas-56287-74-2
56. Ft-0653844
57. D01638
58. D95096
59. A830986
60. Q4689289
61. 2-fluoromethyl-3-(o-tolyl)-6-amino-4(3h)-quinazolinone
62. 6-azanyl-2-(fluoranylmethyl)-3-(2-methylphenyl)quinazolin-4-one
| Molecular Weight | 283.30 g/mol |
|---|---|
| Molecular Formula | C16H14FN3O |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 283.11209024 g/mol |
| Monoisotopic Mass | 283.11209024 g/mol |
| Topological Polar Surface Area | 58.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 439 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
Afloqualone has known human metabolites that include Afloqualone N-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560