


1. Aldoxo
2. Doxo-emch
3. Inno-206
1. Inno-206
2. 1361644-26-9
3. Doxorubicin-emch
4. Doxo-emch
5. Aldoxorubicin [usan]
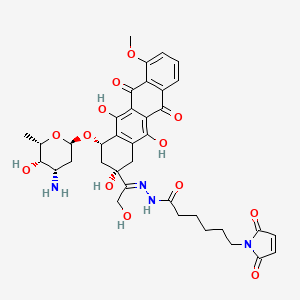
6. N-[(e)-[1-[(2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1h-tetracen-2-yl]-2-hydroxyethylidene]amino]-6-(2,5-dioxopyrrol-1-yl)hexanamide
7. Aldoxorubicin (usan)
8. 151038-96-9
9. C28mv4im0b
10. Aldoxorubicin [inn]
11. Aldoxorubicin [who-dd]
12. Chembl2107818
13. Schembl15221892
14. Dtxsid001098062
15. Ex-a3971
16. Aldoxorubicin;inno 206;inno206
17. Nsc784722
18. Zinc163337436
19. Cs-1186
20. Db06013
21. Nsc-784722
22. Hy-16261
23. D10383
24. (6-maleimidocaproyl)hydrazone Of Doxorubicin
25. (8s)-1-methoxy-6,8alpha,11-trihydroxy-8-[1-[2-[6-(2,5-dioxo-3-pyrroline-1-yl)hexanoyl]hydrazono]-2-hydroxyethyl]-10alpha-(3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyloxy)-7,8,9,10-tetrahydronaphthacene-5,12-dione
26. 1h-pyrrole-1-hexanoic Acid, 2,5-dihydro-2,5-dioxo-, (1-((2s,4s)-4-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl)-2-hydroxyethylidene)hydrazide
27. 1h-pyrrole-1-hexanoic Acid, 2,5-dihydro-2,5-dioxo-, (1-(4-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl)-2-hydroxyethylidene)hydrazide, (2s-cis)-
28. 1h-pyrrole-1-hexanoic Acid, 2,5-dihydro-2,5-dioxo-, (2e)-2-(1-((2s,4s)-4-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl)-2-hydroxyethylidene)hydrazide
29. 1h-pyrrole-1-hexanoic Acid, 2,5-dihydro-2,5-dioxo-, (2e)-2-[1-[(2s,4s)-4-[(3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy]-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-2-naphthacenyl]-2-hydroxyethylidene]hydrazide
30. N'-((1e)-1-((2s,4s)-4-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxohexopyranosyl)oxy)-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl(-2-hydroxyethylidene)-6-(2,5-dioxo-2,5-dihydro-1h-pyrrol-1-yl)hexanohydrazide
31. N'-((e)-1-((2s,4s)-4-(((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl)-2-hydroxyethylidene)-6-(2,5-dioxo-2,5-dihydro-1h-pyrrol-1-yl)hexanehydrazide
| Molecular Weight | 750.7 g/mol |
|---|---|
| Molecular Formula | C37H42N4O13 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 12 |
| Exact Mass | 750.27483741 g/mol |
| Monoisotopic Mass | 750.27483741 g/mol |
| Topological Polar Surface Area | 268 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1510 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in solid tumors.
INNO-206 is the (6-Maleimidocaproyl) hydrazone of doxorubicin. INNO-206 is a prodrug of doxorubicin that binds endogenous albumin after administration. The bound doxorubicin is released in the acidic environment of the tumor cell through cleavage of an acid sensitive linker. In preclinical models, INNO-206 was superior to doxorubicin with regard to antitumor efficacy and toxicity.