


1. Almogran
2. Almotriptan Malate
3. Axert
1. 154323-57-6
2. Axert
3. Almogran
4. Las-31416
5. Las 31416
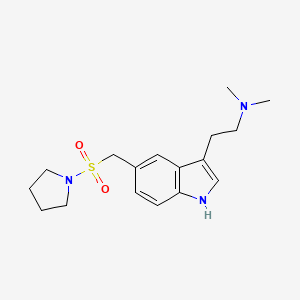
6. N,n-dimethyl-2-(5-((pyrrolidin-1-ylsulfonyl)methyl)-1h-indol-3-yl)ethanamine
7. Almotriptan (usan)
8. 1-(((3-(2-(dimethylamino)ethyl)indol-5-yl)methyl)sulfonyl)pyrrolidine
9. N,n-dimethyl-2-[5-(pyrrolidin-1-ylsulfonylmethyl)-1h-indol-3-yl]ethanamine
10. Nsc-760092
11. Pyrrolidine, 1-(((3-(2-(dimethylamino)ethyl)-1h-indol-5-yl)methyl)sulfonyl)-
12. 1o4xl5sn61
13. Chebi:520985
14. N,n-dimethyl-2-{5-[(pyrrolidin-1-ylsulfonyl)methyl]-1h-indol-3-yl}ethanamine
15. Ncgc00095135-01
16. Almotriptan [usan]
17. Pyrrolidine, 1-[[[3-[2-(dimethylamino)ethyl]-1h-indol-5-yl]methyl]sulfonyl]-
18. Dsstox_cid_24289
19. Dsstox_rid_80142
20. Dsstox_gsid_44289
21. Almotrintan
22. Almotriptan [usan:inn:ban]
23. Dimethyl(2-{5-[(pyrrolidine-1-sulfonyl)methyl]-1h-indol-3-yl}ethyl)amine
24. Unii-1o4xl5sn61
25. Cas-154323-57-6
26. Sr-05000001986
27. Pnu-180638
28. Spectrum_001884
29. Almotriptan [mi]
30. Las 31416almotriptan
31. Spectrum2_000498
32. Spectrum3_001006
33. Spectrum4_001134
34. Spectrum5_001554
35. Almotriptan [inn]
36. Almotriptan [vandf]
37. Schembl1957
38. Chembl1505
39. Almotriptan [who-dd]
40. Bspbio_002731
41. Kbiogr_001647
42. Kbioss_002414
43. Bidd:gt0048
44. Spectrum1505204
45. Spbio_000395
46. Gtpl7110
47. Dtxsid5044289
48. Hy-b0383a
49. Kbio2_002408
50. Kbio2_004976
51. Kbio2_007544
52. Kbio3_001951
53. Zinc18087
54. Hms1922l13
55. Pharmakon1600-01505204
56. Bcp06539
57. Tox21 111444
58. Tox21_111444
59. Ccg-39569
60. Nsc760092
61. Akos015895080
62. Tox21_111444_1
63. Ac-8799
64. Am84500
65. Cs-4530
66. Db00918
67. Nsc 760092
68. Sb19515
69. Ncgc00095135-02
70. Ncgc00095135-03
71. Ncgc00095135-04
72. Ncgc00095135-05
73. Ws-02332
74. Db-064033
75. Ft-0651595
76. 23a576
77. D02824
78. D86136
79. Ab01563047_01
80. Ab01563047_02
81. A809522
82. L000846
83. Q409729
84. Sr-05000001986-1
85. Brd-k67601717-001-02-0
86. 1-[[3-(2-dimethylaminoethyl)-5-indolyl]methanesulphonyl]pyrrolidine
87. N,n-dimethyl-2-(5-((pyrrolidin-1-ylsulfonyl)-methyl)-1h-indol-3-yl)ethanamine
88. N,n-dimethyl-2-[5-(pyrrolidin-1-ylsulfonylmethyl)-1h- Indol-3-yl]-ethanamine
| Molecular Weight | 335.5 g/mol |
|---|---|
| Molecular Formula | C17H25N3O2S |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 335.16674822 g/mol |
| Monoisotopic Mass | 335.16674822 g/mol |
| Topological Polar Surface Area | 64.8 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 483 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Axert |
| PubMed Health | Almotriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | AXERT (almotriptan malate) Tablets contain almotriptan malate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist. Almotriptan malate is chemically designated as 1-[[[3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl]methyl]sulfonyl]pyrrolidi... |
| Active Ingredient | Almotriptan malate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 6.25mg base; eq 12.5mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Axert |
| PubMed Health | Almotriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | AXERT (almotriptan malate) Tablets contain almotriptan malate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist. Almotriptan malate is chemically designated as 1-[[[3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl]methyl]sulfonyl]pyrrolidi... |
| Active Ingredient | Almotriptan malate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 6.25mg base; eq 12.5mg base |
| Market Status | Prescription |
| Company | Janssen Pharms |
For the treatment of acute migraine headache in adults
FDA Label
Almotriptan is a selective 5-hydroxytryptamine receptor subtype agonist indicated for the acute treatment of migraine attacks with or without aura in adults. Almotriptan is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. Almotriptan is an agonist for a vascular 5-hydroxytryptamine receptor subtype (probably a member of the 5-HT1D family) having only a weak affinity for 5-HT1A, 5-HT5A, and 5-HT7 receptors and no significant affinity or pharmacological activity at 5-HT2, 5-HT3 or 5-HT4 receptor subtypes or at alpha1-, alpha2-, or beta-adrenergic, dopamine1,; dopamine2; muscarinic, or benzodiazepine receptors. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that Almotriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels, which may also contribute to the antimigrainous effect of Almotriptan in humans.
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
N02CC05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CC - Selective serotonin (5ht1) agonists
N02CC05 - Almotriptan
Route of Elimination
Almotriptan is eliminated primarily by renal excretion (about 75% of the oral dose), with approximately 40% of an administered dose excreted unchanged in urine. Approximately 13% of the administered dose is excreted via feces, both unchanged and metabolized.
Volume of Distribution
180 to 200 L
Clearance
57 L/h [healthy]
34.2 L/h [moderate renal impairment (creatinine clearance between 31 and 71 mL/min)]
9.8 L/h [severe renal impairment (creatinine clearance between 10 and 30 mL/min)]
Almotriptan has known human metabolites that include 2-hydroxy-almotriptan and methyl(2-{5-[(pyrrolidine-1-sulfonyl)methyl]-1H-indol-3-yl}ethyl)amine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
3-4 hours
Almotriptan binds with high affinity to human 5-HT1B and 5-HT1D receptors leading to cranial blood vessel constriction.