


1. Aluminum Acetate Hydrate
2. Basic Aluminum Acetate Hydrate
3. Burow's Solution
4. Domeboro
1. Domeboro
2. 139-12-8
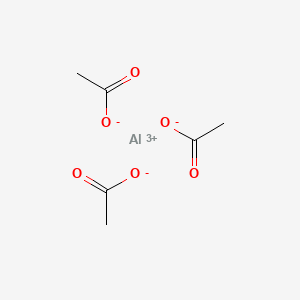
3. Aluminum Triacetate
4. Aluminium Acetate
5. Acetic Acid, Aluminum Salt
6. Aluminum;triacetate
7. Buro-sol
8. Acetic Acid, Aluminum Salt (3:1)
9. 80ehd8i43d
10. Burow Solution
11. Aluminum Acetate Solution
12. Buro-sol Concentrate
13. Hydroxyaluminium Di(acetate)
14. Unii-80ehd8i43d
15. Einecs 205-354-1
16. Aluminium (tri)acetate
17. Aluminium(iii) Triacetate
18. 8006-13-1
19. Aluminum Acetate [ii]
20. Aluminum Acetate [mi]
21. Aluminum Acetate [inci]
22. Aluminum Acetate [vandf]
23. Chembl1201015
24. Dtxsid30890496
25. Aluminium Acetate [mart.]
26. Aluminium Acetate [who-dd]
27. Amy22494
28. Aluminum Acetate [orange Book]
29. Aluminum Acetate [usp Impurity]
30. Db14518
31. Borofair Component Aluminum Acetate
32. Aluminum Acetate Component Of Borofair
33. Q27861942
| Molecular Weight | 204.11 g/mol |
|---|---|
| Molecular Formula | C6H9AlO6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 204.0214514 g/mol |
| Monoisotopic Mass | 204.0214514 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Aluminum Acetate is an astringent. An astrignent is a chemical that tends to shrink or constrict body tissues, usually locally after topical medicinal application. The shrinkage or constriction is through osmotic flow of water (or other fluids) away from the area where the astringent was applied. Astringent medicines cause shrinkage of mucous membranes or exposed tissues and are often used internally to check discharge of blood serum or mucous secretions. This can happen with a sore throat, hemorrhages, diarrhea, or with peptic ulcers. Externally applied astringents, which cause mild coagulation of skin proteins, dry, harden, and protect the skin. Acne sufferers are often advised to use astringents if they have oily skin. Astringents also help heal stretch marks and other scars. Mild astringent solutions are used in the relief of such minor skin irritations as those resulting from superficial cuts, allergies, insect bites, or fungal infections such as athlete's foot.