
1. Scotine
1. (-)-cotinine
2. 486-56-6
3. (s)-cotinine
4. Cotinina
5. Cotininum
6. (s)-(-)-cotinine
7. Cotinine [inn]
8. Cotinine (-)
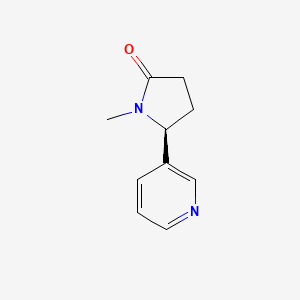
9. (s)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one
10. (5s)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one
11. (s)-1-methyl-5-(3-pyridinyl)-2-pyrrolidinone
12. 2-pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (5s)-
13. Nih 10498
14. (5s)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one
15. Nicotine Related Compound C
16. Chebi:68641
17. K5161x06ll
18. S-(-)-cotinine
19. (-)-cotinine 100 Microg/ml In Acetonitrile
20. Dsstox_cid_27576
21. Dsstox_rid_82428
22. Dsstox_gsid_47576
23. (5~{s})-1-methyl-5-pyridin-3-yl-pyrrolidin-2-one
24. Brd4010
25. Brd-4010
26. (5s)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one ((-)-cotinine)
27. 2-pyrrolidinone, 1-methyl-5-(3-pyridinyl)-, (s)-
28. Smr000449278
29. Cotininum [inn-latin]
30. Sr-01000075768
31. Cotinina [inn-spanish]
32. Unii-k5161x06ll
33. Ccris 7625
34. Hsdb 7805
35. Ncgc00093739-04
36. (5s)-1-methyl-5-(3-pyridinyl)-2-pyrrolidinone
37. Cas-486-56-6
38. Prestwick_134
39. Einecs 207-634-9
40. Mfcd00077696
41. N-methyl-2-(3-pyridyl)-5-pyrrolidone
42. Brn 0083099
43. Spectrum_001984
44. Cotinine [hsdb]
45. Nicotine Ep Impurity C
46. Cotinine [mi]
47. Prestwick0_000082
48. Prestwick1_000082
49. Prestwick2_000082
50. Prestwick3_000082
51. Spectrum3_000700
52. Spectrum4_001793
53. Spectrum5_000465
54. Nicotine Impurity C
55. Bmse000577
56. (-)-cotinine, 98%
57. (s)-1-methyl-5-(3-pyridyl)-2-pyrrolidinone
58. S(-)-1-methyl-5-(3-pyridyl)-2-pyrrolidone
59. (5s)-1-methyl-5-(3-pyridyl)pyrrolidin-2-one
60. Lopac0_000285
61. Schembl49060
62. Bspbio_000004
63. Bspbio_002459
64. Kbiogr_002368
65. Kbioss_002550
66. 5-24-02-00504 (beilstein Handbook Reference)
67. Mls000758262
68. Mls001423950
69. Divk1c_000861
70. Spectrum1500208
71. Spbio_001943
72. (-)-cotinine, >=98%
73. Bpbio1_000006
74. Chembl578211
75. Megxp0_001870
76. Dtxsid1047576
77. Acon1_000202
78. Kbio1_000861
79. Kbio2_002541
80. Kbio2_005109
81. Kbio2_007677
82. Kbio3_001679
83. Ninds_000861
84. Us8609708, 91 Cotinine
85. Hms1568a06
86. Hms1920a14
87. Hms2051a15
88. Hms2091g22
89. Hms2095a06
90. Hms2232f15
91. Hms3260j12
92. Pharmakon1600-01500208
93. Zinc402766
94. (-)-cotinine, Analytical Standard
95. Hy-b1178
96. Tox21_111219
97. Tox21_300615
98. Tox21_500285
99. Bbl102262
100. Bdbm50370573
101. Nih-10498
102. Nsc756704
103. S9339
104. Stl556061
105. Akos007930814
106. Tox21_111219_1
107. (-)-cotinine 1.0 Mg/ml In Methanol
108. Ccg-100799
109. Cs-4787
110. Lp00285
111. Nc00049
112. Nsc-756704
113. Sdccgmls-0066565.p001
114. Sdccgsbi-0050273.p004
115. Idi1_000861
116. Ncgc00093739-08
117. Ncgc00093739-13
118. Ncgc00093739-20
119. Ncgc00254396-01
120. Ncgc00260970-01
121. Ac-35718
122. As-50387
123. Nicotine Impurity C [ep Impurity]
124. Sbi-0050273.p003
125. Am20061246
126. B7277
127. Eu-0100285
128. Nicotine Related Compound C [usp-rs]
129. C 5923
130. P10066
131. Ab00053721_08
132. (5s)-1-methyl-5-pyridin-3-yl-pyrrolidin-2-one
133. A827581
134. Nicotine Related Compound C [usp Impurity]
135. Nicotine Resinate Impurity C [ep Impurity]
136. Q421177
137. Sr-01000075768-1
138. Sr-01000075768-5
139. Sr-01000075768-6
140. (-)-1-methyl-5-(3-pyridyl)-2-pyrrolidinone
141. Brd-k94144010-001-04-8
142. Brd-k94144010-001-05-5
143. Brd-k94144010-001-09-7
144. (-)-cotinine Solution, Drug Standard, 1.0 Mg/ml In Methanol
145. Nicotine Ditartrate Dihydrate Impurity C [ep Impurity]
146. (-)-cotinine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
147. Nicotine Related Compound C, United States Pharmacopeia (usp) Reference Standard
148. Cotinine; Nicotine Usp Related Compound C; (5s)-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one; (s)-(-)-1-methyl-5-(3-pyridiyl)-2- Pyrrolidinone
149. U5h
| Molecular Weight | 176.21 g/mol |
|---|---|
| Molecular Formula | C10H12N2O |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 176.094963011 g/mol |
| Monoisotopic Mass | 176.094963011 g/mol |
| Topological Polar Surface Area | 33.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 205 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Therapeutic Category: Antidepressant. /Experimental Therapy/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 429
/Experimental therapy/ Cotinine is the major metabolite of nicotine in humans, and the substance greatly outlasts the presence of nicotine in the body. Recently, cotinine has been shown to exert pharmacological properties of its own that include potential cognition enhancement, anti-psychotic activity, and cytoprotection. Since the metabolite is generally less potent than nicotine in vivo, we considered whether part of cotinine's efficacy could be related to a reduced ability to desensitize nicotinic receptors as compared with nicotine. Rats freely moving in their home cages were instrumented to allow ongoing measurement of mean arterial blood pressure. The ganglionic stimulant dimethylphenylpiperazinium maximally increased mean arterial blood pressure by 25 mm Hg. Slow (20 min) i.v. infusion of nicotine (0.25-1uLl) produced no change in resting mean arterial blood pressure, but the pressor response to subsequent injection of dimethylphenylpiperazinium was significantly attenuated in a dose-dependent manner by up to 51%. Pre-infusion of equivalent doses of cotinine produced the same maximal degree of inhibition of the response to dimethylphenylpiperazinium. Discrete i.v. injections of nicotine also produced a dose dependent increase in mean arterial blood pressure of up to 43 mm Hg after the highest tolerated dose. In contrast, injection of cotinine produced no significant change in mean arterial blood pressure up to 13 times the highest dose of nicotine. These results illustrate the disconnection between nicotinic receptor activation and receptor desensitization, and they suggest that cotinine's pharmacological actions are either mediated through partial desensitization, or through non-ganglionic subtypes of nicotinic receptors.
PMID:17157984 Buccafusco JJ et al; Neurosci Lett 413 (1): 68-71 (2007).
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Nicotine and its proximate metabolite cotinine are eliminated in part by renal clearance. These compounds are filtered, secreted, and reabsorbed, and the resultant renal clearances are quite variable among individuals and are highly influenced by urine pH. In this study of 139 pairs of twins, we have estimated the genetic and environmental contributions to total renal clearance and net secretory/reabsorptive clearance of nicotine and cotinine. At uncontrolled urine pH both nicotine and cotinine undergo net reabsorption. Additive genetic factors were not important contributors to the variation in total renal clearance of nicotine but played a relatively more substantial role in accounting for the variation in total renal clearance of cotinine (43% of variance). Variations in glomerular filtration rate and the net secretory/reabsorptive clearance of nicotine and cotinine were largely influenced by nonadditive genetic influences (41.5-61% of variance). Earlier research has shown that renal secretory clearance of drugs can be highly heritable, presumably related to genetic variation in transporters. Our study suggests that the renal clearance of drugs that undergo extensive renal reabsorption can be substantially influenced by nonadditive genetic and/or shared environmental factors.
PMID:18388871 Benowitz NL et al; Clin Pharmacol Ther 84 (2): 243-7 (2008).
Assays of metabolised cotinine are considered to be an accurate measure of exposure to cigarette smoke among pregnant women. We investigated the association and differences between the cotinine levels in maternal urine and blood, and the umbilical cord blood of three tobacco exposure groups at different stages of pregnancy. A prospective study was conducted among 398 pregnant women undergoing prenatal care in different trimesters at two medical centres and one regional hospital in central Taiwan. All 398 subjects (including 25 smokers, 191 passive smokers and 182 non-smokers) remained in the study up to the time of delivery; 384 of them delivered singleton live births. Cotinine levels were assayed in the maternal plasma and urine of the mothers at each trimester and in the cord blood of the newborns. All specimens were measured using a sensitive high-performance liquid chromatography. Cotinine concentrations in plasma and urine showed a significant dose-dependent difference among the three groups (non-smoker, passive and active smoker) and a trend that increased with gestation among the pregnant women. Significant correlations between cotinine concentrations in plasma and urine among the pregnant women in each trimester were found. In addition, the level of cotinine in umbilical cord blood was significantly correlated with that in maternal blood at term (r = 0.89, P < 0.001). A pattern of elevated cotinine concentrations in the plasma and urine of pregnant women from the beginning to the end of pregnancy was found, and this correlated significantly with the cotinine levels in the umbilical cord blood.
PMID:18426525 Wu FY et al; Paediatr Perinat Epidemiol 22 (3): 296-301 (2008).
Blood-brain barrier nicotine transfer has been well documented in view of the fact that this alkaloid is a cerebral blood flow marker. However, limited data are available that describe blood-brain barrier penetration of the major tobacco alkaloids after chronic nicotine exposure. This question needs to be addressed, given long-term nicotine exposure alters both blood-brain barrier function and morphology. In contrast to nicotine, it has been reported that cotinine (the major nicotine metabolite) does not penetrate the blood-brain barrier, yet cotinine brain distribution has been well documented after nicotine exposure. Surprisingly, therefore, the literature indirectly suggests that central nervous system cotinine distribution occurs secondarily to nicotine brain metabolism. The aims of the current report are to define blood-brain barrier transfer of nicotine and cotinine in naive and nicotine-exposed animals. Using an in situ brain perfusion model, we assessed the blood-brain barrier uptake of [3H]nicotine and [3H]cotinine in naive animals and in animals exposed chronically to S-(-)nicotine (4.5 mg/kg/day) through osmotic minipump infusion. Our data demonstrate that 1) [3H]nicotine blood-brain barrier uptake is not altered in the in situ perfusion model after chronic nicotine exposure, 2) [3H]cotinine penetrates the blood-brain barrier, and 3) similar to [3H]nicotine, [3H]cotinine blood-brain barrier transfer is not altered by chronic nicotine exposure. To our knowledge, this is the first report detailing the uptake of nicotine and cotinine after chronic nicotine exposure and quantifying the rate of blood-brain barrier penetration by cotinine.
PMID:15845856 Lockman PR et al; J Pharmacol Exp Ther. 2005 Aug;314(2):636-42 (2005).
Nicotine and its primary oxidative metabolites are metabolized in part by glucuronidation. Genetic variation in UGT isoenzymes that catalyze glucuronidation activity suggests that variation in glucuronidation rate is in part genetically determined. The relative contribution of genetic and environmental sources to individual differences in the rate of glucuronidation of nicotine, cotinine, and trans-3'-hydroxycotinine was estimated in a twin study of nicotine pharmacokinetics. Glucuronidation rate was defined using measures that either accounted for variability in renal clearance or assumed the same relative renal clearance of parent drug and glucuronide conjugate across individuals. The former definition resulted in highly correlated nicotine and cotinine glucuronidation measures that were substantially influenced by the combined effect of additive (heritable) and non-additive (dominant and epistatic) genetic effects. These findings suggest that genetic variation in UGT isoenzymes that act in additive and interactive ways is an important determinant of individual variability in nicotine and cotinine metabolism via glucuronidation pathways.
PMID:19803778 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3202423 Lessov-Schlaggar CN et al; Twin Res Hum Genet 12 (5): 507-13 (2009).
Cotinine formation is the major pathway of nicotine metabolism in smokers, and the primary pathway of cotinine metabolism is trans-3'-hydroxylation. trans-3'-Hydroxycotinine and its glucuronide conjugate account for up to 50% of the nicotine metabolites excreted by smokers. Minor metabolites of cotinine excreted by smokers include norcotinine and cotinine N-oxide, each of which account for <5% of the nicotine dose. It has been reported that P450 2A6 is the catalyst of cotinine metabolism. However, we report here that the major product of P450 2A6-catalyzed cotinine metabolism is N-(hydroxymethyl)norcotinine, a previously unknown human metabolite of cotinine. N-(Hydroxymethyl)norcotinine was chemically synthesized, and its stability under the conditions of the enzyme reactions was confirmed. The products of P450 2A6-catalyzed [5-3H]cotinine metabolism were quantified by radioflow HPLC. The identification of N-(hydroxymethyl)norcotinine as the major metabolite was based on HPLC analysis on three unique systems and coelution with N-(hydroxymethyl)norcotinine standard. 5'-Hydroxycotinine and trans-3'-hydroxycotinine were minor products of P450 2A6-catalyzed cotinine metabolism, accounting for 14 and 8% of the total cotinine metabolites, respectively. N-(Hydroxymethyl)norcotinine was a product of cotinine metabolism by the extrahepatic P450, 2A13, but it was a minor one. The major product of P450 2A13-catalyzed cotinine metabolism was 5'-hydroxycotinine, which was formed at twice the rate of trans-3'-hydroxycotinine. The identification of all cotinine metabolites formed by both enzymes was confirmed by LC/MS/MS analysis. Kinetic parameters for cotinine metabolism were determined for P450 2A6 and P450 2A13. This work has confirmed that the major metabolite of cotinine in smokers, trans-3'-hydroxycotinine, is only a minor metabolite of P450 2A6-catalyzed cotinine metabolism.
PMID:16359169 Brown KM et al; Chem Res Toxicol 18 (12): 1792-8 (2005).
Nicotine, a major constituent of tobacco, plays a critical role in smoking addiction. In humans, nicotine is primarily metabolized to cotinine, which is further metabolized to trans-3'-hydroxycotinine. Recently, we have demonstrated that heterologously expressed human CYP2A13 is highly active in the metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a nicotine-derived carcinogen. In the present study, CYP2A13-catalyzed NNK metabolism was found to be inhibited competitively by nicotine and N'-nitrosonornicotine (NNN), suggesting that both nicotine and NNN are also substrates of CYP2A13. We have further demonstrated that human CYP2A13 is indeed an efficient enzyme in catalyzing C-oxidation of nicotine to form cotinine, with the apparent K(m) and V(max) values of 20.2 uM and 8.7 pmol/min/pmol, respectively. CYP2A13 also catalyzes the 3'-hydroxylation of cotinine to form trans-3'-hydroxycotinine, with the apparent K(m) and V(max) values of 45.2 uM and 0.7 pmol/min/pmol, respectively. The importance of CYP2A13-catalyzed nicotine and cotinine metabolism in vivo remains to be determined.
PMID:15528319 He XY et al; Drug Metab Dispos 33 (2): 258-61 (2005). Available as of March 12, 2010:
Nicotine has roles in the addiction to smoking, replacement therapy for smoking cessation, as a potential medication for several diseases such as Parkinson's disease, Alzheimer's disease, and ulcerative colitis. The absorbed nicotine is rapidly and extensively metabolized and eliminated to urine. A major pathway of nicotine metabolism is C-oxidation to cotinine, which is catalyzed by CYP2A6 in human livers. Cotinine is subsequently metabolized to trans-3'-hydroxycotinine by CYP2A6. Nicotine and cotinine are glucuronidated to N-glucuronides mainly by UGT1A4 and partly by UGT1A9. Trans-3'-hydroxycotinine is glucuronidated to O-glucuronide mainly by UGT2B7 and partly by UGT1A9. Approximately 90% of the total nicotine uptake is eliminated as these metabolites and nicotine itself. The nicotine metabolism is an important determinant of the clearance of nicotine. Recently, advances in the understanding of the interindividual variability in nicotine metabolism have been made. There are substantial data suggesting that the large interindividual differences in cotinine formation are associated with genetic polymorphisms of the CYP2A6 gene. Interethnic differences have also been observed in the cotinine formation and the allele frequencies of the CYP2A6 alleles. Since the genetic polymorphisms of the CYP2A6 gene have a major impact on nicotine clearance, its relationships with smoking behavior or the risk of lung cancer have been suggested. The metabolic pathways of the glucuronidation of nicotine, cotinine, and trans-3'-hydroxycotinine in humans would be one of the causal factors for the interindividual differences in nicotine metabolism.
PMID:16141602 Nakajima M, Yokoi T; Drug Metab Pharmacokinet 20 (4): 227-35 (2005).
Cotinine has known human metabolites that include 5'-hydroxycotinine, Cotinine N-glucuronide, Norcotinine, and trans-3'-hydroxycotinine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Cotinine levels in infants are higher than in older children or adults exposed to the same reported quantity of environmental tobacco smoke. One hypothesis to explain this difference is that the urinary elimination half-life of cotinine is different between infants and older children. Urine was collected at admission, 12, 24 and 48 hr, cotinine levels were subsequently measured and then standardized by correcting for creatinine excretion. Urinary elimination half-life of cotinine was calculated in 31 infants and 23 older children. The median half-life was 28.3 hr (range 6.3-258.5 hr) in infants, and 27.14 hr (range 9.7-99.42 hr) in older children. A Mann-Whitney U test showed no significant difference in the median half-life of cotinine between the two age groups (P = 0.18). Multivariate linear regression analysis demonstrated no significant relationship between half-life of cotinine and corrected cotinine level (P = 0.24). Our results support the hypothesis that higher cotinine levels in infants is due to greater exposure, rather than slower metabolism of cotinine.
PMID:10101746 Leong JW et al; Pulm Pharmacol Ther. 1998;11(4):287-90 (1998).
The current study examined selected factors of ethnicity, menthol cigarette preference, body composition and alcohol-use history on cotinine half-life in 6 days of smoking abstinence in African American and Caucasian women. A 7-day inpatient protocol was conducted in the General Clinical Research Center, in which day 1 was ad lib smoking and days 2-7 were smoking abstinence (n = 32). Plasma cotinine was measured every 8 h throughout. Average cotinine half-life was 21.3 hr, similar to previously reported 18-20 hr. Three women exhibited >14 ng/mL cotinine after 136 hr of smoking abstinence. Host factors explaining 52.0% of variance in cotinine half-life and associated with longer half-life were being an African American menthol smoker, fewer years of alcohol use and greater lean body mass. Among menthol smokers, baseline cotinine level and cotinine half-life were not significantly different in Caucasian and African American women.
PMID:12521401 Ahijevych KL et al; Nicotine Tob Res 4 (4): 423-31 (2002).
The nicotine metabolite cotinine is an abundant long-lived bio-active compound that may contribute to the overall physiological effects of tobacco use. Although its mechanism of action in the central nervous system has not been extensively investigated, cotinine is known to evoke dopamine release in the nigrostriatal pathway through an interaction at nicotinic receptors (nAChRs). Because considerable evidence now demonstrates the presence of multiple nAChRs in the striatum, the present experiments were done to determine the subtypes through which cotinine exerts its effects in monkeys, a species that expresses similar densities of striatal alpha4beta2* (nAChR containing the alpha4 and beta2 subunits, but not alpha3 or alpha6) and alpha3/alpha6beta2* (nAChR composed of the alpha3 or alpha6 subunits and beta2) nAChRs. Competition binding studies showed that cotinine interacts with both alpha4beta2* and alpha3/alpha6beta2* nAChR subtypes in the caudate, with cotinine IC(50) values for inhibition of 5-[(125) I]iodo-3-[2(S)-azetinylmethoxy]pyridine-2HCl ([(125)I]A-85380) and (125)I-alpha-conotoxinMII binding in the micromolar range. This interaction at the receptor level is of functional significance because cotinine stimulated both alpha4beta2* and alpha3/alpha6beta2* nAChR [(3)H]dopamine release from caudate synaptosomes. Our results unexpectedly showed that nicotine evokes [(3)H]dopamine release from two alpha3/alpha6beta2* nAChR populations, one of which was sensitive to cotinine and the other was not. This cotinine-insensitive subtype was only present in the medial caudate and was preferentially lost with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal damage. In contrast, cotinine and nicotine elicited equivalent levels of alpha4beta2* nAChR-mediated dopamine release. These data demonstrate that cotinine functionally discriminates between two alpha3/alpha6beta2* nAChRs in monkey striatum, with the cotinine-insensitive alpha3/alpha6beta2* nAChR preferentially vulnerable to nigrostriatal damage.
PMID:18305015 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3134143 O'Leary K et al; J Pharmacol Exp Ther 325 (2): 646-54 (2008).
Cotinine is the major metabolite of nicotine in humans, and the substance greatly outlasts the presence of nicotine in the body. Recently, cotinine has been shown to exert pharmacological properties of its own that include potential cognition enhancement, anti-psychotic activity, and cytoprotection. Since the metabolite is generally less potent than nicotine in vivo, we considered whether part of cotinine's efficacy could be related to a reduced ability to desensitize nicotinic receptors as compared with nicotine. Rats freely moving in their home cages were instrumented to allow ongoing measurement of mean arterial blood pressure. The ganglionic stimulant dimethylphenylpiperazinium maximally increased mean arterial blood pressure by 25 mm Hg. Slow (20 min) i.v. infusion of nicotine (0.25-1umol) produced no change in resting mean arterial blood pressure, but the pressor response to subsequent injection of dimethylphenylpiperazinium was significantly attenuated in a dose-dependent manner by up to 51%. Pre-infusion of equivalent doses of cotinine produced the same maximal degree of inhibition of the response to dimethylphenylpiperazinium. Discrete i.v. injections of nicotine also produced a dose dependent increase in mean arterial blood pressure of up to 43 mm Hg after the highest tolerated dose. In contrast, injection of cotinine produced no significant change in mean arterial blood pressure up to 13 times the highest dose of nicotine. These results illustrate the disconnection between nicotinic receptor activation and receptor desensitization, and they suggest that cotinine's pharmacological actions are either mediated through partial desensitization, or through non-ganglionic subtypes of nicotinic receptors.
PMID:17157984 Buccafusco JJ et al; Neurosci Lett 413 (1): 68-71 (2007).
The aim of the present study was to clarify whether cotinine affects the release of catecholamines from the isolated perfused rat adrenal gland, and to establish the mechanism of its action, in comparison with the response of nicotine. Cotinine (0.3-3 mM), when perfused into an adrenal vein for 60 min, inhibited catecholamines secretory responses evoked by ACh (5.32 mM), DMPP (a selective neuronal nicotinic agonist, 100 uM for 2 min) and McN-A-343 (a selective muscarinic M1-agonist, 100 uM for 2 min) in dose- and time-dependent manners. However, cotinine did not affect catecholamines secretion by high K+ (56 mM). Cotinine itself also failed to affect basal catecholamines output. Furthermore, in the presence of cotinine (1 mM), catecholamines secretory responses evoked by Bay-K-8644 (an activator of L-type Ca2+ channels, 10 uM) and cyclopiazonic acid (an inhibitor of cytoplasmic Ca2+-ATPase, 10 uM) were relative time-dependently attenuated. However, nicotine (30 uM), given into the adrenal gland for 60 min, initially rather enhanced catecholamines secretory responses evoked by ACh and high K+, followed by the inhibition later, while it time-dependently depressed the catecholamines release evoked by McN-A-343 and DMPP. Taken together, these results suggest that cotinine inhibits greatly catecholamines secretion evoked by stimulation of cholinergic (both nicotinic and muscarinic) receptors, but does fail to affect that by the direct membrane-depolarization. It seems that this inhibitory effect of cotinine may be exerted by the cholinergic blockade, which is associated with blocking both the calcium influx into the rat adrenal medullary chromaffin cells and Ca2+ release from the cytoplasmic calcium store. It also seems that there is a big difference in the mode of action between cotinine and nicotine in the rat adrenomedullary catecholamines secretion.
PMID:14560925 Koh YY et al; Arch Pharm Res 26 (9): 747-55 (2003).