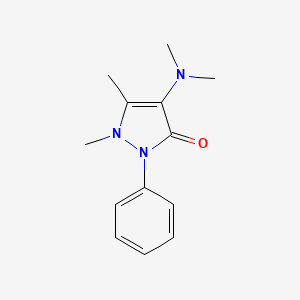

1. Amidazophen
2. Amidophen
3. Amidophenazon
4. Amidopyrine
5. Aminofenazone
6. Aminophenazone
7. Dimethyl N Aminoantipyrine
8. Dimethyl-n-aminoantipyrine
9. Dimethylaminoantipyrine
10. Dimethylaminophenazone
11. Dipyrine
12. Eufibron
1. Aminophenazone
2. Amidopyrine
3. 58-15-1
4. 4-dimethylaminoantipyrine
5. Dipyrine
6. Amidopyrazoline
7. Amidazophene
8. Pyramidon
9. Aminofenazone
10. Amidazophen
11. Amidophen
12. Aminopyrin
13. Brufaneuxol
14. Itamidone
15. Dereuma
16. Febron
17. Dimethylaminoazophene
18. Dimethylaminophenazone
19. Amidophenazone
20. Aminophenazon
21. Dimethylaminoantipyrine
22. Amidofebrin
23. Amidofen
24. Amidopyrin
25. Anafebrina
26. Eufibron
27. Netsusarin
28. Pyramidone
29. Amidazofen
30. Piromidina
31. Dimapyrin
32. Dipirin
33. Dipyrin
34. Febrinina
35. Hyparon
36. Novamidon
37. Piramidon
38. Pirazon
39. Piridol
40. Polinalin
41. Pyradone
42. 4-(dimethylamino)antipyrine
43. Mamallet-a
44. 4-dimethylaminophenazone
45. 4-dimethylamino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one
46. Dimethylamino-analgesine
47. Dimethylaminophenyldimethylpyrazolone
48. Dimethylaminophenazon
49. Aminofenazona
50. Aminophenazonum
51. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one
52. (dimethylamino)phenazone
53. Antipyrine, 4-(dimethylamino)-
54. Aminopyrine [jan]
55. 4-dimethylamino-1-phenyl-2,3-dimethylpyrazolone
56. 1,5-dimethyl-4-dimethylamino-2-phenyl-3-pyrazolone
57. 1-phenyl-2,3-dimethyl-4-dimethylaminopyrazol-5-one
58. 2,3-dimethyl-4-dimethylamino-1-phenyl-5-pyrazolone
59. 4-dimethylamino-2,3-dimethyl-1-phenyl-5-pyrazolone
60. Mamallet A
61. 3h-pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-
62. Dimethylaminophenyldimethylpyrazolin
63. Aminophenazone [inn]
64. 3-keto-1,5-dimethyl-4-dimethylamino-2-phenyl-2,3-dihydropyrazole
65. Nsc-4993
66. 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-3h-pyrazol-3-one
67. 1-phenyl-2,3-dimethyl-4-(dimethylamino)-5-pyrazolone
68. 4-(dimethylamino)-1,5-dimethyl-2-phenylpyrazol-3-one
69. Mls002154195
70. 3-pyrazolin-5-one, 4-(dimethylamino)-2,3-dimethyl-1-phenyl-
71. Chebi:160246
72. 01704yp3mo
73. 3h-pyrazol-3-one,4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-
74. Aminopyrine (jan)
75. Aminophenazone (inn)
76. Cas-58-15-1
77. Ncgc00016257-04
78. Amidazophenum
79. Amidopyrinum
80. Aminopyrinum
81. Smr001216566
82. Dsstox_cid_504
83. 4,4-dimethylaminophenazone
84. Dsstox_rid_75627
85. Dsstox_gsid_20504
86. 1-phenyl-2,3-dimethyl-4-dimethylaminopyrazolone-5
87. Aminophenazon [german]
88. Aminofenazone [italian]
89. Aminofenazona [inn-spanish]
90. Aminophenazonum [inn-latin]
91. Dimethylaminophenazon [german]
92. Ccris 2907
93. Hsdb 2135
94. Aminopyrine [jan:nf]
95. Sr-05000001741
96. Nsc 4993
97. Einecs 200-365-8
98. Mfcd00003142
99. Piramidone
100. Unii-01704yp3mo
101. Dimethylaminophenyldimethylpyrazone
102. Prestwick_14
103. Aminophenazone-[d6]
104. Aminopyrine [mi]
105. Prestwick0_000088
106. Prestwick1_000088
107. Prestwick2_000088
108. Prestwick3_000088
109. Aminopyrine [hsdb]
110. 4-(dimethylamino)phenazone
111. Cid_6009
112. Timtec1_000737
113. 3-keto-1,3-dihydropyrazole
114. Oprea1_080671
115. Schembl26293
116. Bspbio_000016
117. 4-n,n-dimethylaminoantipyrine
118. 4-dimethylamino-2,3-dimethyl-1-phenylpyrazol-5-one
119. Spbio_001955
120. Aminophenazone [mart.]
121. Bpbio1_000018
122. Chembl288470
123. Aminophenazone [who-dd]
124. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-pyrazol-3-one
125. Dtxsid7020504
126. Bdbm74258
127. Zinc57115
128. Nsc4993
129. 4-(dimethylamino)-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one
130. Hms1536b11
131. Hms1568a18
132. Hms2092e10
133. Hms2095a18
134. Hms3039c06
135. Hms3652g13
136. Hms3712a18
137. Pharmakon1600-01500621
138. Hy-b0533
139. Tox21_110333
140. Tox21_201544
141. Tox21_302830
142. Nsc757388
143. S3209
144. Stl356033
145. Tocainidehydrochloride(125mg)
146. Akos001590378
147. Tox21_110333_1
148. Aminophenazone 100 Microg/ml In Water
149. Ccg-103785
150. Db01424
151. Nsc-757388
152. Ncgc00016257-01
153. Ncgc00016257-02
154. Ncgc00016257-03
155. Ncgc00016257-05
156. Ncgc00016257-06
157. Ncgc00016257-07
158. Ncgc00016257-10
159. Ncgc00090878-01
160. Ncgc00090878-02
161. Ncgc00090878-03
162. Ncgc00175173-01
163. Ncgc00175173-02
164. Ncgc00256436-01
165. Ncgc00259094-01
166. 3-pyrazolin-5-one,3-dimethyl-1-phenyl-
167. Ac-12025
168. Sbi-0207073.p001
169. Wln: T5nnvj A1 Br& Dn1&1 E1
170. Db-053161
171. B1883
172. Sw196332-3
173. Aminophenazone 100 Microg/ml In Acetonitrile
174. C07539
175. D00556
176. Q416503
177. 1,5-dimethyl-2-phenyl-4-dimethylamino-3-pyrazolone
178. Sr-05000001741-1
179. Sr-05000001741-3
180. Sr-05000001741-4
181. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-3-pyrazolone
182. Brd-k12568846-001-04-7
183. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-3-pyrazolin-3-one
184. 4-dimethylaminoantipyrine, Reactive Nitrogen Species Scavenger
185. Metamizole Sodium Monohydrate Impurity D [ep Impurity]
186. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-1h-pyrazol-3(2h)-one
187. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one #
188. 4-(dimethylamino)-1,5-dimethyl-2-phenyl-2,3-dihydro-1h-pyrazol-3-one
189. 4-dimethylamino-1,5-dimethyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one
190. Aminophenazone (4-dimethylamino-1,5-dimethyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one)
191. 4-dimethylamino-1,5-dimethyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one (4,4-dimethylaminophenazone)
| Molecular Weight | 231.29 g/mol |
|---|---|
| Molecular Formula | C13H17N3O |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 231.137162174 g/mol |
| Monoisotopic Mass | 231.137162174 g/mol |
| Topological Polar Surface Area | 26.8 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
IN SOME CASES OF PROLONGED INTRACTABLE FEVER, AS IN HODGKIN'S DISEASE & PERIARTERITIS NODOSA, AMINOPYRINE IS CAPABLE OF CONTROLLING /FEVER/ & MAY BE JUSTIFIED. /SRP: EFFECTIVE AND PREVIOUSLY USED IN THE US AS AN ANTIPYRETIC, ANALGESIC AND ANTI-INFLAMMATORY AGENT/.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 348
ANALGESIC & ANTIPYRETIC. IT IS EFFECTIVE IN RELIEF OF PAIN IN NEURALGIA, DYSMENORRHEA, RHEUMATISM, & SIMILAR PAINFUL CONDITIONS. ...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1053
MEDICATION (VET): ANALGESIC, ANTIPYRETIC. TO RELIEVE MINOR ACHES, JOINT & MUSCULAR PAINS, & REDUCE ELEVATED TEMP. RELIEVES PAIN & POSSIBLE TOXIC EFFECTS IN INDIGESTIONS OF CATTLE.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 14
For more Therapeutic Uses (Complete) data for AMINOPYRINE (7 total), please visit the HSDB record page.
IF USED AT ALL IN TREATMENT OF INTRACTABLE FEVER, AMINOPYRINE...SHOULD BE EMPLOYED ONLY AFTER SAFER DRUGS & OTHER MEASURES HAVE PROVEN INEFFECTIVE, & ONLY WITH PROPER SUPERVISION & MONITORING.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 348
LEUKOCYTE COUNT & DIFFERENTIAL SHOULD BE CHECKED DURING ADMIN OF LARGE DOSES OR CONTINUED USE OF SMALL DOSES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1053
IN RARE INDIVIDUALS, INSTEAD OF FRANK AGRANULOCYTOSIS, EACH ADMIN OF AMINOPYRINE PRODUCES SHARP FALL IN TOTAL LEUKOCYTE COUNT ASSOC WITH SEVERE CHILL, SPIKING FEVER, HEADACHE, & PAIN IN MUSCLES & JOINTS; ATTACK IS OVER WITHIN FEW HR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 347
VET: MAY STIMULATE METABOLISM OF OTHER DRUGS (CHLORINATED HYDROCARBONS, BARBITURATES, PHENYLBUTAZONE, MEPROBAMATE) BY LIVER MICROSOMES.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 14
For more Drug Warnings (Complete) data for AMINOPYRINE (6 total), please visit the HSDB record page.
4. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG BETWEEN 1 TEASPOON & 1 OZ FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-239
Formerly widely used as an antipyretic and analgesic in rheumatism, neuritis, and common colds. Currently used to measure total body water.
Aminophenazone exhibits analgesic, anti-inflammatory, and antipyretic properties.
N02BB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N02BB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N02BB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N02BB03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BB - Pyrazolones
N02BB03 - Aminophenazone
IT IS ABSORBED RAPIDLY FOLLOWING ORAL ADMIN...EXCRETED IN URINE UNCHANGED OR CONJUGATED WITH GLUCURONIC & SULFURIC ACIDS.
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 363
...SLIGHTLY BOUND TO PLASMA PROTEIN...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 347
THREE METABOLITES OF AMINOPYRINE WERE DETECTED IN THE URINE, SERUM, AND SALIVA OF HEALTH SUBJECTS AFTER INGESTION OF AMINOPYRINE.
WERNER M, WERNER D; AGENTS ACTIONS SUPPL AAS 10 (TRENDS INFLAMMATION RES 2): 99 (1982)
IMMEDIATELY AFTER INJECTION OF AMINOPYRINE-(14)C INTO RATS A UNIFORM DISTRIBUTION OF RADIOACTIVITY IN THE BODY WAS RECORDED. AFTER 30 MINUTES, A PREFERENTIAL LOCALIZATION OF RADIOACTIVITY WAS FOUND IN THE NASAL MUCOSA AND LIVER.
BRITTEBO EB; ACTA PHARMACOL TOXICOL 51 (3): 227 (1982)
...URINARY METABOLITE OF AMINOPYRINE HAS BEEN IDENTIFIED AS 4-ACETYLAMINO-3-METHYL-1-PHENYLPYRAZOLONE IN TREATED SUCKLINGS, INFANTS, & ADULT. ... FORMATION.../OF THIS METABOLITE/ IMPLICATES EXHAUSTIVE OXIDATIVE N-DEALKYLATION OF.../AMINOPYRINE/ FOLLOWED BY ACETYLATION OF RELEASED AMINO-GROUP.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 186
...BIOTRANSFORMATION OF AMINOPYRINE. MAN & DOG EXCRETE RUBAZONIC ACID...& N-METHYLRUBAZONIC ACID...WHOSE FORMATION INVOLVES NUMBER OF METABOLIC STEPS. FOLLOWING FORMATION OF 4-HYDROXYANTIPYRINE, THIS METABOLITE IS N-DEMETHYLATED... & DEHYDROGENATED TO HYPOTHETICAL DIKETO INTERMEDIATE...LATTER CMPD...UNDERGOES FINAL COUPLING...WITH ANOTHER METABOLITE, 4-AMINOANTIPYRINE...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 165
CIRCADIAN RHYTHMS...OFTEN SEEN WITHIN A GIVEN ANIMAL SPECIES. VARIATION IN RATE OF METABOLISM IS OFTEN CORRELATED WITH VARIATIONS IN ENDOCRINE FUNCTION AS INFLUENCED BY THE LIGHT-DARK CYCLE TO WHICH THE ANIMAL IS EXPOSED. ...IN VITRO METABOLISM OF...AMINOPYRINE...BY THE HEPATIC CYTOCHROME P450 MONOOXYGENASE SYSTEM OF RATS IS VARIABLE DEPENDING ON THE TIME OF DAY OF SACRIFICE OF THE ANIMAL.
Doull, J., C.D. Klaassen, and M. D. Amdur (eds.). Casarett and Doull's Toxicology. 2nd ed. New York: Macmillan Publishing Co., 1980., p. 67
AMINOPHENAZONE AND ITS METABOLITES; 4-AMINOANTIPYRINE, N-ACETYLAMINOANTIPYRINE AND 4-FORMYLAMINOANTIPYRINE WERE DETECTED IN STUDIES ON HEALTHY SUBJECTS.
WERNER M, WERNER D; AGENTS ACTIONS SUPPL, AAS 10 (TRENDS INFLAMMATION RES 2): 99 (1982)
Aminophenazone is metabolized very slowly by normal newborn babies. In older infants, a higher amount of exhaled 13-CO2 is observed.
VET: AMIDOPYRINE IS KNOWN TO CAUSE AGRANULOCYTOSIS IN MAN, BUT IT HAS BEEN FOUND IMPOSSIBLE TO REPRODUCE THIS EFFECT IN THE DOG & OTHER LABORATORY ANIMALS.
Clarke, M. L., D. G. Harvey and D. J. Humphreys. Veterinary Toxicology. 2nd ed. London: Bailliere Tindall, 1981., p. 93