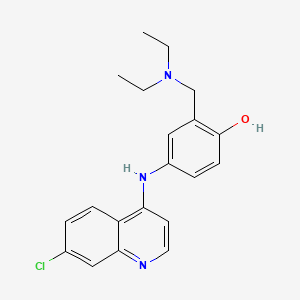

1. Amodiachin
2. Amodiaquin
3. Amodiaquine Hydrochloride
4. Camoquin
5. Camoquine
6. Flavoquine
7. Hydrochloride, Amodiaquine
1. 86-42-0
2. Amodiaquin
3. Flavoquine
4. Camoquin
5. Camoquine
6. Camochin
7. Camoquinal
8. Miaquin
9. Amodiaquinum
10. Amodiaquine, Ring-closed
11. Cam-aq1
12. Amodiachinum
13. Amodiaquina
14. Sunoquine
15. 4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)methyl]phenol
16. Cam-aqi
17. Amodiaquine Hydrochloride
18. Sn 10,751
19. 4-((7-chloroquinolin-4-yl)amino)-2-((diethylamino)methyl)phenol
20. S. N. 10751
21. Phenol, 4-[(7-chloro-4-quinolinyl)amino]-2-[(diethylamino)methyl]-
22. Nsc 13453
23. Chebi:2674
24. 7-chloro-4-(3-diethylaminomethyl-4-hydroxyanilino)quinoline
25. Nsc-13453
26. 7-chloro-4-(3-diethylaminomethyl-4-hydroxyphenylamino)quinoline
27. Chembl682
28. 4-((7-chloro-4-quinolinyl)amino)-2-((diethylamino)methyl)phenol
29. Phenol, 4-((7-chloro-4-quinolinyl)amino)-2-((diethylamino)methyl)-
30. Gnf-pf-5648
31. Amodiachin
32. 4-[(7-chloro-4-quinolinyl)amino]-2-[(diethylamino)methyl]phenol
33. Nsc13453
34. Amodiaquin (dihydrochloride Dihydrate)
35. 220236ed28
36. Amodiaquinum [inn-latin]
37. Cqa
38. Amodiaquina [inn-spanish]
39. Amodiaquine Usp24
40. 4-((7-chloro-4-quinolyl)amino)-.alpha.-(diethylamino)-o-cresol
41. Einecs 201-669-3
42. Brn 0300962
43. Alphaquine
44. Amdaquine
45. Amoquin
46. Amodiaquine [usp:inn:ban]
47. Ccris 8486
48. O-cresol, 4-((7-chloro-4-quinolyl)amino)-.alpha.-(diethylamino)-
49. O-cresol, 4-[(7-chloro-4-quinolyl)amino]-.alpha.-(diethylamino)-
50. Quinoline, 7-chloro-4-((3-((diethylamino)methyl)-4-hydroxyphenyl)amino)-
51. Quinoline, 7-chloro-4-[[3-[(diethylamino)methyl]-4-hydroxyphenyl]amino]-
52. 4-((7-chloro-4-quinolyl)amino)-alpha-(diethylamino)-o-cresol
53. 4-[(7-chloroquinolin-4-yl)amino]-2-(diethylaminomethyl)phenol
54. Hsdb 7457
55. 2aou
56. Unii-220236ed28
57. Amodiaquin [mi]
58. Wr-002977
59. Amodiaquine (usp/inn)
60. Prestwick0_000309
61. Prestwick1_000309
62. Prestwick2_000309
63. Prestwick3_000309
64. Amodiaquine [inn]
65. Amodiaquine [hsdb]
66. Epitope Id:131784
67. Amodiaquine [vandf]
68. Amodiaquine [mart.]
69. Oprea1_019229
70. Schembl44152
71. Amodiaquine [who-dd]
72. Amodiaquine [who-ip]
73. Bspbio_000278
74. 5-22-10-00283 (beilstein Handbook Reference)
75. Mls001304065
76. Amodiaquine; Flavoquine
77. Spbio_002497
78. Bpbio1_000306
79. Dtxsid2022597
80. Gtpl10018
81. Hy-b1322a
82. 4-[(7-chloro-4-quinolyl)amino]-2-(diethylaminomethyl)phenol
83. Amodiaquine [usp Impurity]
84. Hms2236k03
85. Hms3372m02
86. Zinc608172
87. Amodiaquine [usp Monograph]
88. Amodiaquinum [who-ip Latin]
89. O-cresol, 4-((7-chloro-4-quinolyl)amino)-alpha-(diethylamino)-
90. Bdbm50041457
91. S5948
92. Akos000538864
93. Ccg-103317
94. Db00613
95. Ncgc00017063-04
96. Ncgc00017063-07
97. Ncgc00017063-13
98. Ncgc00244901-01
99. Ncgc00244901-04
100. Ac-13295
101. Smr000718769
102. Db-056928
103. Cs-0013340
104. Ft-0622359
105. Ft-0662117
106. Sj000110703
107. Vu0472676-1
108. C07626
109. D02922
110. 552a927
111. A841639
112. A936101
113. Q239569
114. W-104068
115. 4-(7-chloroquinolin-4-ylamino)-2-((diethylamino)methyl)phenol
116. 4-[(7-chloro-4-quinolinyl)amino]-.alpha.-(diethylamino)-o-cresol
117. 2-((bc20h12d10cln3ois(ethyl-d5)amino)methyl)-4-((7-chloroquinolin-4-yl)amino)phenol
118. 1021496-19-4
| Molecular Weight | 355.9 g/mol |
|---|---|
| Molecular Formula | C20H22ClN3O |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 355.1451400 g/mol |
| Monoisotopic Mass | 355.1451400 g/mol |
| Topological Polar Surface Area | 48.4 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 406 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
THERAP CAT: Antimalarial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 95
There are very few recent data on the in vivo susceptibility of P. ovale and P. malariae to antimalarials. Both species are regarded as very sensitive to chloroquine, although there is a single recent report of chloroquine resistance in P. malariae. Experience indicates that P. ovale and P. malariae are also susceptible to amodiaquine, mefloquine and the artemisinin derivatives.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
Summary of recommendations on the treatment of uncomplicated vivax malaria: Amodiaquine (30 mg base/kg bw divided over 3 days as 10 mg/kg bw single daily doses) combined with primaquine should be given for chloroquine-resistant vivax malaria.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
/Indicated/ for /the/ treatment of acute malarial attacks in non-immune subjects. It is at least as effective as chloroquine, and is effective against some chloroquine-resistant strains, although resistance to amodiaquine has been reported.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
Agranulocytosis Associated with the Use of Amodiaquine for Malaria Prophylaxis Seven cases of agranulocytosis associated with the use of amodiaquine (Camoquine) among British travelers have recently been reported (1). Sixteen additional cases of agranulocytosis from Western Europe associated with the use of amodiaquine have recently been reported to the drug manufacturer, and two U.S. cases have been reported to CDC. Twenty-three of these 25 cases occurred in 1985 or 1986, and seven are reported to have been fatal. Among 20 cases for which the duration of amodiaquine prophylaxis is known, usage ranged from 3 weeks to 24 weeks. In all but four of the 25 cases, amodiaquine was used at the appropriate dosage (adults 400 mg base per week) for prophylaxis. Fourteen of the patients are known to have used another antimalarial drug concurrently for prophylaxis ... It is now apparent that any possible prophylactic advantage that amodiaquine may afford is not justified by the possible risk of agranulocytosis associated with the use of the drug. CDC, therefore, no longer recommends that amodiaquine be used for prophylaxis.
PMID:3081784 CDC; Morbidity and Mortality Weekly Report 35 (10): 165-166 (1986)
Because amodiaquine may concentrate in the liver, the drug should be used with caution in patients with hepatic disease or alcoholism, and in patients receiving hepatotoxic drugs.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
Children are especially sensitive to 4-aminoquinoline derivatives. Because of the narrow margin between the therapeutic and toxic concentrations in children, amodiaquine should not be administered parenterally in this age group.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
Amodiaquine is contraindicated in patients who are hypersensitive /to 4-aminoquinoline derivatives/.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
For more Drug Warnings (Complete) data for AMODIAQUINE (14 total), please visit the HSDB record page.
It is likely that the fatal dose for amodiaquine would be similar to that of chloroquine phosphate (2 to 3 g, adult) since amodiaquine appears to completely parallel the adverse effects of those seen with chloroquine when equivalent doses are used.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
For treatment of acute malarial attacks in non-immune subjects.
Amodiaquine, a 4-aminoquinoline similar to chloroquine in structure and activity, has been used as both an antimalarial and an anti-inflammatory agent for more than 40 years. Amodiaquine is at least as effective as chloroquine, and is effective against some chloroquine-resistant strains, although resistance to amodiaquine has been reported. The mode of action of amodiaquine has not yet been determined. 4-Aminoquinolines depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension. They depress respiration and cause diplopia, dizziness and nausea.
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01B - Antimalarials
P01BA - Aminoquinolines
P01BA06 - Amodiaquine
Absorption
Rapidly absorbed following oral administration.
Amodiaquine hydrochloride is readily absorbed from the gastrointestinal tract. It is rapidly converted in the liver to the active metabolite desethylamodiaquine, which contributes nearly all of the antimalarial effect (10). There are insufficient data on the terminal plasma elimination half-life of desethylamodiaquine. Both amodiaquine and desethylamodiaquine have been detected in the urine several months after administration.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
After oral administration amodiaquine hydrochloride is rapidly absorbed...
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
After oral administration of amodiaquine (600 mg) to 7 healthy adult males ... The peak concentration of amodiaquine was 32 +/- 3 ng/mL at 0.5 +/- 0.03 hr. The peak concentrations of amodiaquine in whole blood and packed cells were 60 +/- 10 and 42 +/- 6 ng/mL respectively, reached at 0.5+/- 0.1hr in both. Thereafter the concentration of amodiaquine declined rapidly, and was detectable for no more than 8 hr.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
Mean peak plasma concentration of the metabolite (desethylamodiaquine) was 181 +/- 26 ng/mL. Times to peak for whole blood and packed cells were 2.2 +/- 0.5 and 3.6 +/- 1.1 hr respectively
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Avalable from: https://www.inchem.org/pages/pims.html as of July 24, 2006.
For more Absorption, Distribution and Excretion (Complete) data for AMODIAQUINE (10 total), please visit the HSDB record page.
Hepatic biotransformation to desethylamodiaquine (the principal biologically active metabolite) is the predominant route of amodiaquine clearance with such a considerable first pass effect that very little orally administered amodiaquine escapes untransformed into the systemic circulation.
... Amodiaquine hydrochloride ... undergoes rapid and extensive metabolism to desethylamodiaquine which concentrates in blood cells. It is likely that desethylamodiaquine, not amodiaquine, is responsible for most of the observed antimalarial activity, and that the toxic effects of amodiaquine after oral administration may in part be due to desethylamodiaquine.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
When amodiaquine is given orally relatively little of the parent compound is present in the blood. Hepatic biotransformation to desethylamodiaquine (the principal biologically active metabolite) is the predominant route of amodiaquine clearance with such a considerable first pass effect that very little orally administered amodiaquine escapes untransformed into the systemic circulation.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
The hepatic metabolism of the antimalarial drug amodiaquine was investigated in order to gain further insight into the postulated metabolic causation of the hepatotoxicity, which restricts the use of the drug. After intraportal administration (54 mumol/kg) to the anaesthetized rat, the drug was excreted in bile (23 +/- 3% dose over 5 h; mean +/- SD, n = 6) primarily as thioether conjugates. After intraportal administration, 20% of the dose was excreted into urine over 24 h as parent compound and products of N-dealkylation and oxidative deamination. Desethylamodiaquine accumulated in liver, but was not a substrate for bioactivation as measured by biliary elimination of a glutathione adduct. Prior administration of ketoconazole, an inhibitor of P450, reduced biliary excretion by 50% and effected a corresponding decrease in the amount of drug irreversibly bound to liver proteins. This indicated a role for P450 in the bioactivation of amodiaquine to a reactive metabolite that conjugates with glutathione and protein. De-ethylation and irreversible binding were observed in vitro using male rat liver microsomes, and were again inhibited by ketoconazole. However, no such binding was observed with human (six individuals) hepatic microsomes despite extensive turnover of amodiaquine to desethylamodiaquine. Amodiaquine quinoneimine underwent rapid reduction in the presence of either human or rat liver microsomes. Therefore in vitro studies may underestimate the bioactivation of amodiaquine in vivo. These data indicate that the extent of protein adduct formation in the liver will depend on the relative rates of oxidation of amodiaquine and reduction of its quinoneimine. This in turn may be a predisposing factor in the idiosyncratic hepatotoxicity associated with amodiaquine. Substitution of a fluorine for the phenolic hydroxyl group in amodiaquine blocked bioactivation of the drug in vivo. Insertion of an N-hydroxyethyl function enabled partial clearance of amodiaquine and its deshydroxyfluoro analogue via O-glucuronidation and altered the balance between phase I oxidation and direct phase II conjugation of amodiaquine.
PMID:7618347 Jewell H et al; Xenobiotica 25 (2): 199-217 (1995)
Amodiaquine (AQ) metabolism to N-desethylamodiaquine (DEAQ) is the principal route of disposition in humans. Using human liver microsomes and two sets of recombinant human cytochrome P450 isoforms (from lymphoblastoids and yeast) /the authors/ performed studies to identify the CYP isoform(s) involved in the metabolism of AQ. CYP2C8 was the main hepatic isoform that cleared AQ and catalyzed the formation of DEAQ. The extrahepatic P450s, 1A1 and 1B1, also cleared AQ and catalyzed the formation of an unknown metabolite M2. The K(m) and V(max) values for AQ N-desethylation were 1.2 microM and 2.6 pmol/min/pmol of CYP2C8 for recombinant CYP2C8, and 2.4 microM and 1462 pmol/min/mg of protein for human liver microsomes (HLMs), respectively. Relative contribution of CYP2C8 in the formation of DEAQ was estimated at 100% using the relative activity factor method. Correlation analyses between AQ metabolism and the activities of eight hepatic P450s were made on 10 different HLM samples. Both the formation of DEAQ and the clearance of AQ showed excellent correlations (r(2) = 0.98 and 0.95) with 6alpha-hydroxylation of paclitaxel, a marker substrate for CYP2C8. The inhibition of DEAQ formation by quercetin was competitive with K(i) values of 1.96 for CYP2C8 and 1.56 microM for HLMs. Docking of AQ into the active site homology models of the CYP2C isoforms showed favorable interactions with CYP2C8, which supported the likelihood of an N-desethylation reaction. These data show that CYP2C8 is the main hepatic isoform responsible for the metabolism of AQ. The specificity, high affinity, and high turnover make AQ desethylation an excellent marker reaction for CYP2C8 activity.
PMID:11805197 Li XQ et al; J Pharmacol Exp Ther. 300 (2):399-407 (2002)
5.2 ± 1.7 (range 0.4 to 5.5) minutes
Amodiaquine 600 mg was given by mouth, the apparent terminal half-life of amodiaquine was 5.2 + 1.7 (range 0.4 to 5.5) minutes and the geometric mean of the estimated elimination phase half-lives was 2.1 (range 0.5 to 5.7) hours.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html
The mechanism of plasmodicidal action of amodiaquine is not completely certain. Like other quinoline derivatives, it is thought to inhibit heme polymerase activity. This results in accumulation of free heme, which is toxic to the parasites. The drug binds the free heme preventing the parasite from converting it to a form less toxic. This drug-heme complex is toxic and disrupts membrane function.
Amodiaquine is a Mannich base 4-aminoquinoline with a mode of action similar to that of chloroquine. It is effective against some chloroquine-resistant strains of P. falciparum, although there is cross-resistance.
WHO; WHO Guidelines for the Treatment of Malaria (2006). Available from, as of July 31, 2006: https://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
The 4-aminoquinoline derivatives appear to bind to nucleoproteins and interfere with protein synthesis in susceptible organisms; the drugs intercalate readily into double-stranded DNA and inhibit both DNA and RNA polymerase. In addition, the drugs apparently concentrate in parasite digestive vacuoles, increase the pH of the vacuoles, and interfere with the parasite's ability to metabolize and utilize erythrocyte hemoglobin. Plasmodial forms that do not have digestive vacuoles and do not utilize hemoglobin, such as exoerythrocytic forms, are not affected by /these medications/.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 859
The 4-aminoquinoline derivatives ... have anti-inflammatory activity; however, the mechanism(s) of action of the drugs in the treatment of rheumatoid arthritis and lupus erythematosus has not been determined. /4-aminoquinoline derivatives/ reportedly antagonizes histamine in vitro, has antiserotonin effects, and inhibits prostaglandin effects in mammalian cells presumably by inhibiting conversion of arachidonic acid to prostaglandin F2.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 859
The mode of action of amodiaquine has not yet been determined. 4-Aminoquinolines depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension; they depress respiration and cause diplopia, dizziness and nausea.
WHO; Poisons Information Monographs (PIMs) 030: Amodiaquine. Available from, as of July 24, 2006: https://www.inchem.org/pages/pims.html