

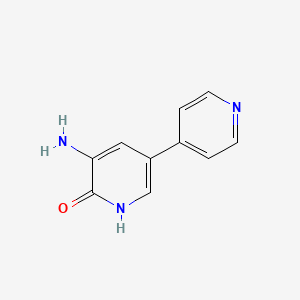

1. 5-amino-(3,4'-bipyridine)-6(1h)-one
2. Amrinon
3. Cordemcura
4. Inocor
5. Win 40680
6. Win-40680
7. Win40680
8. Wincoram
1. 60719-84-8
2. Inamrinone
3. Wincoram
4. Inocor
5. Cordemcura
6. Cartonic
7. Amcoral
8. Amrinonum [inn-latin]
9. Vesistol
10. 5-amino-[3,4'-bipyridin]-6(1h)-one
11. Amrinona [inn-spanish]
12. 3-amino-5-(4-pyridinyl)-2(1h)-pyridinone
13. Win-40680
14. Win 40680
15. 3-amino-5-pyridin-4-yl-1h-pyridin-2-one
16. 5-amino-(3,4'-bipyridin)-6(1h)-one
17. Awd 08-250
18. [3,4'-bipyridin]-6(1h)-one, 5-amino-
19. Amrinone [inn]
20. 5-amino-3,4'-bipyridin-6(1h)-one
21. Amrinone Lactate
22. Mls000069829
23. 5-amino(3,4'-bipyridin)-6(1h)-one
24. Nsc-759805
25. (3,4'-bipyridin)-6(1h)-one, 5-amino-
26. Smr000058850
27. Chembl12856
28. Jut23379tn
29. C01ce01
30. 3-amino-5-pyridin-4-ylpyridin-2-ol
31. 3-amino-5-(pyridin-4-yl)pyridin-2-ol
32. 5-amino[3,4'-bipyridin]-6(1h)-one
33. Ncgc00164379-01
34. Amrinona
35. Amrinonum
36. Cas-60719-84-8
37. Dsstox_cid_2603
38. Dsstox_rid_76655
39. Dsstox_gsid_22603
40. Inamrinone (usp)
41. Amcoral (tn)
42. Ccris 3794
43. 3-amino-5-(pyridin-4-yl)pyridin-2(1h)-one
44. Amrinone (jan/inn)
45. Inamrinone [usan:usp]
46. Einecs 262-390-0
47. Brn 0744819
48. Unii-jut23379tn
49. Prestwick_44
50. Amrinone,(s)
51. Mfcd00083228
52. Spectrum_001350
53. Amrinone [jan]
54. Amrinone [mi]
55. Amrinone [vandf]
56. Opera_id_1054
57. Prestwick0_000800
58. Prestwick1_000800
59. Prestwick2_000800
60. Prestwick3_000800
61. Spectrum2_001980
62. Spectrum3_000956
63. Spectrum4_001069
64. Spectrum5_000999
65. Inamrinone [usan]
66. Amrinone [mart.]
67. Inamrinone [vandf]
68. Amrinone [usp-rs]
69. Amrinone [who-dd]
70. Cid_3698
71. Schembl44012
72. Bspbio_000940
73. Kbiogr_001398
74. Kbioss_001830
75. 5-25-15-00181 (beilstein Handbook Reference)
76. Mls001074083
77. Divk1c_000136
78. Spectrum1503084
79. Spbio_002139
80. Spbio_002879
81. Bpbio1_001034
82. Chebi:2686
83. Gtpl7202
84. Dtxsid9022603
85. Schembl13457017
86. Bdbm34651
87. Hms500g18
88. Kbio1_000136
89. Kbio2_001830
90. Kbio2_004398
91. Kbio2_006966
92. Kbio3_002052
93. Inamrinone [usp Impurity]
94. Ninds_000136
95. Hms1570o22
96. Hms2097o22
97. Hms2234f12
98. Hms3264e16
99. Hms3369j10
100. Hms3714o22
101. Inamrinone [usp Monograph]
102. Pharmakon1600-01503084
103. Bcp12764
104. Hy-b1294
105. Zinc8673078
106. Tox21_112110
107. Tox21_301847
108. Ccg-39487
109. Nsc759805
110. Stk590307
111. Akos005512516
112. Tox21_112110_1
113. Db01427
114. Ks-5178
115. Nsc 759805
116. Cid 5281003
117. Idi1_000136
118. Ncgc00016896-01
119. Ncgc00016896-02
120. Ncgc00016896-03
121. Ncgc00016896-04
122. Ncgc00016896-06
123. Ncgc00095984-01
124. Ncgc00255137-01
125. Ac-12186
126. Ac-33137
127. Ba166404
128. 5-amino(3,4'-bipyridine)-6-(1h)-one
129. 5-amino-(3,4?-bipyridin)-6(1h)-one
130. 3-amino-5-(4-pyridinyl)-2(1h)pyridinone
131. 3-azanyl-5-pyridin-4-yl-1h-pyridin-2-one
132. Cs-0013064
133. Ft-0601595
134. 5-amino-3,4'-bipyridyl-6(1h)-one
135. C75837
136. D00231
137. Ab00052312-04
138. Ab00052312_05
139. 3-amino-5-(4-pyridinyl)-1,2-dihydro-2-pyridone
140. 719a848
141. A832852
142. Q422724
143. Sr-00000002445
144. Sr-00000002445-2
145. W-105234
146. 3-amino-5-(pyridin-4-yl)-1,2-dihydropyridin-2-one
147. Brd-k45924332-001-04-4
148. Brd-k45924332-001-07-7
149. Amrinone, United States Pharmacopeia (usp) Reference Standard
150. 216151-27-8
| Molecular Weight | 187.20 g/mol |
|---|---|
| Molecular Formula | C10H9N3O |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 187.074561919 g/mol |
| Monoisotopic Mass | 187.074561919 g/mol |
| Topological Polar Surface Area | 68 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 301 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of congestive heart failure.
Amrinone is a positive inotropic cardiotonic with vasodilator properties, phosphodiesterase inhibitory activity, and the ability to stimulate calcium ion influx into the cardiac cell.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Phosphodiesterase 3 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 3. (See all compounds classified as Phosphodiesterase 3 Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CE - Phosphodiesterase inhibitors
C01CE01 - Amrinone
Route of Elimination
The primary route of excretion in man is via the urine as both inamrinone and several metabolites (N-glycolyl, N-acetate, O-glucuronide and N-glucuronide).
Volume of Distribution
1.2 L/kg [normal volunteers]
Hepatic.
5 to 8 hours
Amrinone is a phosphodiesterase inhibitor (PDE3), resulting in increased cAMP and cGMP which leads to an increase in the calcium influx like that caused by beta-agonists resulting in increased inotropic effect.