
1. Amsa
2. Amsa P D
3. Amsa P-d
4. Amsa Pd
5. Amsacrina
6. Amsidine
7. Amsidyl
8. Cain Acridine
9. Cain's Acridine
10. Cains Acridine
11. M-amsa
12. Meta Amsa
13. Meta-amsa
14. Nsc 141549
15. Nsc 156303
16. Nsc 249992
17. Nsc-141549
18. Nsc-156303
19. Nsc-249992
20. Nsc141549
21. Nsc156303
22. Nsc249992
23. Sn 11841
24. Sn-11841
25. Sn11841
1. 51264-14-3
2. M-amsa
3. Amsidine
4. Amsidyl
5. Acridinylanisidide
6. Lamasine
7. Amekrin
8. Amsacrina
9. Mamsa
10. Acridinyl Anisidide
11. Amsacrinum
12. Amsine
13. 4'-(9-acridinylamino)methanesulfon-m-anisidide
14. Amsidil
15. 4'-(9-acridinylamino)-3'-methoxymethanesulfonanilide
16. Sn-11841
17. Ci-880
18. Nsc-249992
19. 4'-(9-acridinylamino)methanesulphon-m-anisidide
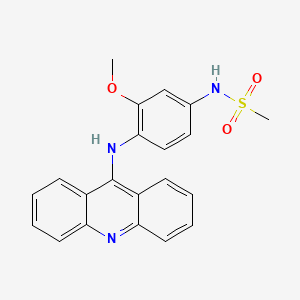
20. N-(4-(acridin-9-ylamino)-3-methoxyphenyl)methanesulfonamide
21. N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide
22. Nsc249992
23. Nsc 156303
24. Nsc 249992
25. Nsc-156303
26. 4'-(9-acridinylamino)methanesulfon-meta-anisidide
27. Sn 21429
28. M-amsa;acridinyl Anisidide
29. Methanesulfonamide, N-[4-(9-acridinylamino)-3-methoxyphenyl]-
30. Chembl43
31. N-(4-(9-acridinylamino)-3-methoxyphenyl)methanesulfonamide
32. N-[4-(9-acridinylamino)-3-methoxyphenyl]methanesulfonamide
33. Methanesulfonamide, N-(4-(9-acridinylamino)-3-methoxyphenyl)-
34. 00dpd30soy
35. Chebi:2687
36. Methanesulfonanilide, 4'-(9-acridinylamino)-3'-methoxy-
37. Meta-amsacrine
38. Methanesulfon-m-anisidide, 4'-(9-acridinylamino)-
39. Nci-249992
40. Sn-21429
41. N-[4-(acridin-9-ylamino)-3-(methyloxy)phenyl]methanesulfonamide
42. Amsacrinum [inn-latin]
43. Amsacrina [inn-spanish]
44. M-amsa Hydrochloride; Acridinyl Anisidide Hydrochloride
45. Amecrin
46. Mls002153376
47. Namsa
48. Amsidyl (tn)
49. Ccris 1027
50. Hsdb 7087
51. Amsacrine (usan/inn)
52. Smr000875352
53. Einecs 257-094-3
54. Ci 880
55. Unii-00dpd30soy
56. Amsa, M-
57. Brn 0500176
58. Amsacrine [usan:inn:ban]
59. Amsacrine [inn]
60. Amsacrine [mi]
61. Amsacrine [hsdb]
62. Amsacrine [iarc]
63. Amsacrine [usan]
64. Amsacrine [vandf]
65. Lopac-a-9809
66. Amsacrine [mart.]
67. Ncimech_000607
68. Amsacrine [who-dd]
69. Neuro_000118
70. Schembl4047
71. Lopac0_000154
72. 5-22-11-00030 (beilstein Handbook Reference)
73. Mls006010099
74. Cid_148673
75. Dtxsid4022604
76. Bdbm87351
77. Hms3748e05
78. N-[4-(9-acridinylamino)-3-methoxy-phenyl]methanesulfonamide
79. N-[4-(acridin-9-ylamino)-3-methoxy-phenyl]methanesulfonamide
80. Bca26414
81. Bcp08958
82. Zinc3812923
83. Ccg-35555
84. Mfcd00242748
85. Akos015917522
86. Cs-1942
87. Db00276
88. Sdccgsbi-0050142.p003
89. Ncgc00015113-01
90. Ncgc00015113-02
91. Ncgc00015113-03
92. Ncgc00015113-04
93. Ncgc00093644-10
94. Ncgc00162077-01
95. As-11665
96. Hy-13551
97. Nci60_001995
98. Smr000857391
99. Wln: T C666 Bnj Imr Bo1 Dmsw1
100. Db-082052
101. Ft-0708980
102. C01553
103. C75400
104. D02321
105. 4'-(9-acridinylamino)methanesulfonyl M-anisidide
106. 301a154
107. 4'-(9-acridinylamino)-methylsulfonyl-m-anisidine
108. A918341
109. Q2784004
110. Amsa;m-amsa;ci-880;sn-11841;acridinyl Anisidide
111. N-[4-(9-acridinylamino)-3-methoxyphenyl]methanesulfonamide;hydrochloride
112. N-[4-(acridin-9-ylamino)-3-methoxy-phenyl]methanesulfonamide;hydrochloride
113. N-{4-[(acridin-9-yl)amino]-3-methoxyphenyl}methanesulfonamide
114. Asw
| Molecular Weight | 393.5 g/mol |
|---|---|
| Molecular Formula | C21H19N3O3S |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 393.11471265 g/mol |
| Monoisotopic Mass | 393.11471265 g/mol |
| Topological Polar Surface Area | 88.7 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 601 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cytostatic agent with antiviral and immunosuppressive properties.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 100
Amsacrine is indicated for induction of remission in acute adult leukemia refractory to conventional therapy. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 129
118 patients with acute leukemias, including initial, relapsed and refractory cases, were treated with domestic Amsacrine (m-AMSA), singly or combined with other drugs. The total CR rate was 39.5% in ALL and 38.8% in ANLL, the response rate was 47.5% for both types of acute leukemias. The CR rate of relapsed and refractory ALL and ANLL treated with combination chemotherapy including domestic m-AMSA was 30.8% and 46.2% respectively. Domestic m-AMSA was similar to the foreign product and many other antitumor drugs in side effects and toxicity. The pharmacokinetics parameters of the drugs, C12h/C6h,K21 and Cmax were correlated with the therapeutic effectiveness
Zhonghua Nei Ke Za Zhi 32(2): 80-84 (1993)
The synthetic aminoacridine derivative amsacrine (m-AMSA) is capable of preventing DNA from serving as a template in replication and DNA synthesis. This mechanism of action is similar to that of anthracyclines, but clinical evidence suggests the lack of cross-resistance. The recommended dosage in patients with solid tumors is 90-120 mg/sq m intravenously every 3-4 weeks. Despite the initial encouraging reports from experimental models, m-AMSA has shown no real impact in the treatment of patients with a wide variety of solid tumors. In relapsed acute nonlymphocytic leukemia, 20-30% of patients will achieve complete remission. An increased remission rate is obtained when m-AMSA is combined with other agents, especially with high-dose cytosine arabinoside, with a complete remission rate of 50-60% in relapsed patients. Currently, several phase III trials are evaluating m-AMSA combinations against daunorubicin-containing regimens in patients with previously untreated acute leukemia. The potential role of these regimens in this disease remains to be defined.
PMID:2582401 Hornedo J et al; Pharmacotherapy 5(2): 78-90 (1985)
For more Therapeutic Uses (Complete) data for AMSACRINE (7 total), please visit the HSDB record page.
Human systemic effects by intravenous route: nausea or vomiting, thrombosis distant from the injection site, and bone marrow changes.
Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 54
Although very little information is available regarding distribution of antineoplastic agents into breast milk, breast-feeding is not recommended during chemotherapy because of the potential risks to the infant (adverse effects, mutagenicity, carcinogenicity).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 130
No information is available on the relationship of age to the effects of amsacrine in geriatric patients. However, elderly patients are more likely to have age-related renal function impairment, which may require adjustment of dosage in patients receiving amsacrine.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 130
The bone marrow depressant effects of amsacrine may result in an increased incidence of microbial infection, delayed healing, and gingival bleeding. Dental work, whenever possible, should be completed prior to initiation of therapy or deferred until blood counts have returned to normal. Patients should be instructed in proper oral hygiene, including caution in use of regular toothbrushes, dental floss, and toothpicks.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 130
For more Drug Warnings (Complete) data for AMSACRINE (19 total), please visit the HSDB record page.
For treatment of acute myeloid leukaemia.
Amsacrine is an aminoacridine derivative that is a potent intercalating antineoplastic agent. It is effective in the treatment of acute leukemias and malignant lymphomas, but has poor activity in the treatment of solid tumors. It is frequently used in combination with other antineoplastic agents in chemotherapy protocols. It produces consistent but acceptable myelosuppression and cardiotoxic effects.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Intercalating Agents
Agents that are capable of inserting themselves between the successive bases in DNA, thus kinking, uncoiling or otherwise deforming it and therefore preventing its proper functioning. They are used in the study of DNA. (See all compounds classified as Intercalating Agents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX01 - Amsacrine
Absorption
Poorly absorbed
Volume of distribution (VolD) -- 1.67 L/kg. Amsacrine does not significantly penetrate into the CNS
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 129
Elimination: Renal: 35% of the dose is excreted by the kidneys within 72 hours after administration (20% as intact drug). Biliary: Amsacrine is also eliminated by biliary excretion.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 130
In cancer patients, amsacrine undergoes biphasic elimination, with a distribution half-life of 0.25-1.6 hours and an elimination half-time of 4.7-9 hours. The total plasma clearance rate is 200-300 ml/min per sq m, and the apparent distribution volume is 70-110 l/sq m, suggesting concentration in tissues. During a 1 hour injusion of amasacrine at 90-200 mg/sq m, the peak plasma concentration was 10-15 umol/l.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 321 (2000)
Although not fully reported, early trials in which amsacrine was given orally failed to reach the maximum tolerated dose, as shown by lack of toxicity even at doses as high as 500 mg/sq m per day, suggesting incomplete or erratic absorption. In subsequent studies, the intravenous route was used, with which the maximum tolerated dose in patients with solid tumors is 100-150 mg/sq m when administered over 1-3 hours.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 321 (2000)
After intravenous administration of (14)C amsacrine to mice and rats, > 50% of the radiolabel was excreted in bile within the first 2 hours, and the bile:plasma ratio was > 400:1; 74% of an intravenous dose was excreted in the feces of mice with 72 hours. These studies demonstrate the importance of the liver in clearance of amsacrine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 322 (2000)
Extensive, primarily hepatic, converted to glutathione conjugate.
Oxidative metabolism of the anti-cancer drug amsacrine 4'-(9-acridinylamino) methane-sulphan-m-anisidide has been suggested to account for its cytotoxicity. However, enzymes capable of oxidizing it in non-hepatic tissue have yet to be identified. A potential candidate, that may be relevant to the metabolism of amsacrine in blood and its action in myeloid leukaemias and myelosuppression, is the haem enzyme myeloperoxidase. We have found that the purified human enzyme oxidizes amsacrine to its quinone diimine, either directly or through the production of hypochlorous acid. In comparison, the 4-methyl-5-methylcarboxamide derivative of amsacrine, CI-921 9-[[2-methoxy-4[(methylsulphonyl)-amino]phenyl]amino)-N, 5-dimethyl-4-acridine carboxamide, reacted poorly with myeloperoxidase, although it was oxidized by hypochlorous acid. Detailed studies of the mechanism by which myeloperoxidase oxidizes amsacrine revealed that the semiquinone imine free radical is a likely intermediate in this reaction. Oxidation of amsacrine analogues indicated that factors other than their reduction potential determine how readily they are metabolized by myeloperoxidase. Both amsacrine and CI-921 inhibited production of hypochlorous acid by myeloperoxidase. CI-921 acted by trapping the enzyme as the inactive redox intermediate compound II. Amsacrine inhibited by a different mechanism that may involve conversion of myeloperoxidase to compound III, which is also unable to oxidize Cl-. The susceptibility of amsacrine to oxidation by myeloperoxidase indicates that this reaction may contribute to the cytotoxicity of amsacrine toward neutrophils, monocytes and their precursors.
PMID:1333205 Kettle AJ et al; Biochem Pharmacol 44(9): 1731-1738 (1992)
In mouse bile, 5'- and 6'-glutathione conjugates were present in roughly equal amounts and accounted for 70% of the excreted biliary radiolabel after administration of radiolabelled amsacrine. In rats, the principal biliary metabolite was the 5'-gutathione conjugate, which accounted for 80% of the excreted radiolabel within the first 90 minutes and > 50% of the administered dose over 3 hours. The 6'-conjugate was also subsequently identified in rat bile. In rat liver microsomes and human neutrophils, intermediate oxidation products have been identified as N1'-methanesulfonyl-N4'-(9-acridinyl)-3'-methoxy-2',5'-cyclohexadience-1',4'-dii mine and 3'-methoxy-4'-(9-acridinylamino-2'5'-cyclohexadien-1'-one.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 322 (2000)
8-9 hours
Amsacrine binds to DNA through intercalation and external binding. It has a base specificity for A-T pairs. Rapidly dividing cells are two to four times more sensitive to amsacrine than are resting cells. Amsacrine appears to cleave DNA by inducing double stranded breaks. Amsacrine also targets and inhibits topoisomerase II. Cytotoxicity is greatest during the S phase of the cell cycle when topoisomerase levels are at a maximum.
Amsacrine binds to DNA through intercalation and external binding and has base specificity for A-T pairs. Cycling cells are two to four times more sensitive to amsacrine than are resting cells. Cells initially in S and G2 phases are grossly delayed in their capacity for normal progression, leading to an accumulation of cells in the S phase, followed at later times by arrest in the G2 phase.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 129
Cytotoxicity of several classes of antitumor DNA intercalators is thought to result from disturbance of DNA metabolism following trapping of the nuclear enzyme DNA topoisomerase II as a covalent complex on DNA. Here, molecular interactions of the potent antitumor drug amsacrine (m-AMSA), an inhibitor of topoisomerase II, within living K562 cancer cells have been studied using surface-enhanced Raman (SER) spectroscopy. The work is based on data of the previously performed model SER experiments dealing with amsacrine/DNA, drug/topoisomerase II and drug/DNA/topoisomerase II complexes in aqueous buffer solutions. The SER data indicated two kinds of amsacrine interactions in the model complexes with topoisomerase II alone or within ternary complex: non-specific (via the acridine moiety) and specific to the enzyme conformation (via the side chain of the drug). These two types of interactions have been both revealed by the micro-SER spectra of amsacrine within living K562 cancer cells. Our data suppose the specific interactions of amsacrine with topoisomerase II via the side chain of the drug (particular feature of the drug/topoisomerase II and ternary complexes) to be crucial for its inhibitory activity.
Chourpa I et al; FESBS Letters 397(1): 61-64 (1996)