

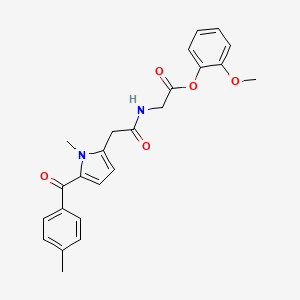

1. 2-methoxyphenyl 1-methyl-5-(4-methylbenzoylpyrrol)-2-acetamidoacetate
2. Amtolmetin Guacyl
3. Amtolmetineguacil
4. Artromed
5. Med 15
6. Med-15
7. Med15
8. St 679
9. St-679
1. 87344-06-7
2. 2-methoxyphenyl 2-(2-(1-methyl-5-(4-methylbenzoyl)-1h-pyrrol-2-yl)acetamido)acetate
3. Artromed
4. Amtolmetin Guacyl
5. St 679
6. St-679
7. Med 15
8. Amtolmetin Guacil [inn]
9. Med-15
10. Finrid
11. Med15
12. 104076-16-6
13. 2-methoxyphenyl 1-methyl-5-p-methylbenzoylpyrrole-2-acetoamidoacetate
14. 323a00cro9
15. (2-methoxyphenyl) 2-[[2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetyl]amino]acetate
16. Amtolmetin Guacil (inn)
17. N-((1-methyl-5-p-toluoylpyrrol-2-yl)acetyl)glycine O-methoxyphenyl Ester
18. 2-methoxyphenyl (2-(1-methyl-5-(4-methylbenzoyl)-1h-pyrrol-2-yl)acetyl)glycinate
19. Eufans
20. Amtolmetine Guacil
21. Amtolmetina Guacilo
22. Amtolmetinum Guacilum
23. Amtolmetine Guacil [inn-french]
24. Amtolmetina Guacilo [inn-spanish]
25. Amtolmetinum Guacilum [inn-latin]
26. Unii-323a00cro9
27. Amtolmetin-guacil
28. Artromed (tn)
29. N-((1-methyl-5-(4-methylbenzoyl)-1h-pyrrol-2-yl)acetyl)glycine 2-methoxyphenyl Ester
30. Schembl24555
31. Mls006010220
32. Amtolmetin Guacil [mi]
33. Chembl1766570
34. Dtxsid50236291
35. Amtolmetin Guacil [mart.]
36. Chebi:135678
37. St679
38. Zinc596929
39. Amtolmetin Guacil [who-dd]
40. Bcp28767
41. Mfcd00866153
42. Stl451031
43. Akos015895070
44. Glycine, N-((1-methyl-5-(4-methylbenzoyl)-1h-pyrrol-2-yl)acetyl)-, 2-methoxyphenyl Ester
45. Glycine, N-((5-benzoyl-1-methyl-1h-pyrrol-2-yl)acetyl)-, 2-methoxyphenyl Ester
46. N-[2-[1-methyl-5-(4-methylbenzoyl)-1h-pyrrol-2-yl]acetyl]glycine 2-methoxyphenyl Ester
47. As-30751
48. Smr004701308
49. Hy-107320
50. Cs-0028135
51. Ft-0659812
52. D07453
53. 344a067
54. A842102
55. Sr-01000945065
56. Q4748890
57. Sr-01000945065-1
58. (2-methoxyphenyl)2-[[2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetyl]amino] Acetate
59. 2-methoxyphenyl 2-(1-methyl-5-(4-methylbenzoyl)-1h-pyrrole-2-acetylamino)acetate
60. 2-methoxyphenyl 2-{2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetamido}acetate
61. 2-methoxyphenyl N-({1-methyl-5-[(4-methylphenyl)carbonyl]-1h-pyrrol-2-yl}acetyl)glycinate
62. 2-methoxyphenyl2-(1-methyl-5-(4-methylbenzoyl)-1h-pyrrole-2-acetylamino)acetate
63. (2-methoxyphenyl) 2-[2-[1-methyl-5-(4-methylphenyl)carbonyl-pyrrol-2-yl]ethanoylamino]ethanoate
64. 2-[[2-[1-methyl-5-[(4-methylphenyl)-oxomethyl]-2-pyrrolyl]-1-oxoethyl]amino]acetic Acid (2-methoxyphenyl) Ester
65. Amtolmetin Guacil; 2-methoxyphenyl 2-(1-methyl-5-(4-methylbenzoyl)-1h-pyrrole-2-acetylamino)acetate
| Molecular Weight | 420.5 g/mol |
|---|---|
| Molecular Formula | C24H24N2O5 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 420.16852187 g/mol |
| Monoisotopic Mass | 420.16852187 g/mol |
| Topological Polar Surface Area | 86.6 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 632 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)