
1. Cc 10004
2. Cc-10004
3. Cc10004
4. Otezla
1. 608141-41-9
2. Otezla
3. Cc-10004
4. Cc 10004
5. Apremilast (cc-10004)
6. Cc10004
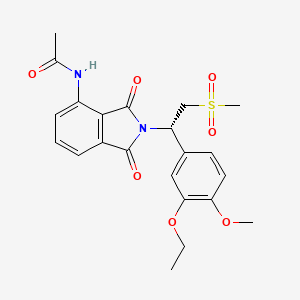
7. (s)-n-(2-(1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide
8. Chebi:78540
9. Up7qbp99pn
10. Chembl514800
11. N-{2-[(1s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-1,3-dioxo-2,3-dihydro-1h-isoindol-4-yl}acetamide
12. N-[2-[(1s)-1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-1,3-dioxoisoindol-4-yl]acetamide
13. (s)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione
14. N-{2-[(1s)-1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1h-isoindol-4-yl}acetamide
15. (+)-n-(2-((1s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxo- 2,3-dihydro-1h-isoindol-4-yl)acetamide
16. Apremilast [usan]
17. Apremilast [usan:inn]
18. Unii-up7qbp99pn
19. Apremilastum
20. Mfcd18782607
21. Hsdb 8221
22. Apremilast- Bio-x
23. Otezla (tn)
24. Apremilast [mi]
25. Apremilast [inn]
26. Apremilast [jan]
27. Apremilast (jan/usan)
28. Apremilast [vandf]
29. Apremilast [who-dd]
30. Schembl302992
31. Amy371
32. Gtpl7372
33. Apremilast [orange Book]
34. Dtxsid30976289
35. Ex-a336
36. Cas:608141-41-9;apremilast
37. 608141-44-2
38. Bcp03783
39. Bcp25283
40. Bdbm50248919
41. S8034
42. Zinc30691736
43. Akos016339660
44. Bcp9000311
45. Ccg-269336
46. Cs-0671
47. Db05676
48. Fk-0727
49. Ac-27650
50. Ba164215
51. Hy-12085
52. Bcp0726000109
53. Sw219856-1
54. D08860
55. Q2858961
56. (s)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione(apremilast)
57. (s)-n-{2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methanesulfonyl-ethyl]-1,3-dioxo-2,3-dihydro-1h-isoindol-4-yl}-acetamide
58. 4-[[5 Pound Not7-dihydroxy-2 Pound Not2-dimethyl-8-(2-methylpropanoyl)chromen-6-yl]methy L]-3 Pound Not5-dihydroxy-6 Pound Not6-dimethyl-2-(2-methylpropanoyl)cyclohexa-2 Pound Not4-dien- 1-one
59. A9l
60. Acetamide, N-(2-((1s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl)- 2,3-dihydro-1,3-dioxo-1h-isoindol-4-yl)-
61. Acetamide, N-[2-[(1s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1h-isoindol-4-yl]-
62. N-[2-[(1s)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1h-isoindol-4-yl]acetamide
| Molecular Weight | 460.5 g/mol |
|---|---|
| Molecular Formula | C22H24N2O7S |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 460.13042228 g/mol |
| Monoisotopic Mass | 460.13042228 g/mol |
| Topological Polar Surface Area | 128 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 825 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal; Phosphodiesterase Inhibitors
National Library of Medicine's Medical Subject Headings. Apremilast. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Otezla is indicated for the treatment of adult patients with active psoriatic arthritis. /Included in US product label/
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
Otezla is indicated for the treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. /Included in US product label/
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of December 31, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
EXPL THER /The purpose of this study is/ to evaluate the efficacy and safety of an oral phosphodiesterase 4 inhibitor, apremilast, in treatment of ankylosing spondylitis (AS) by monitoring symptoms and signs in a pilot study including exploratory investigation of effects of PDE4 inhibition on blood biomarkers of bone biology. In this double-blind, placebo-controlled, single-centre, Phase II study, patients with symptomatic AS with active disease on MRI were randomized to apremilast 30 mg BID or placebo over 12 weeks. Bath Indices were monitored serially. Patients were followed for 4 weeks after stopping medication. Bone biomarkers were assessed at baseline and day 85. 38 subjects were randomised and 36 subjects completed the study. Although the primary end-point (change in BASDAI at week 12) was not met, apremilast was associated with numerically greater improvement from baseline for all clinical assessments compared with placebo with mean change in BASDAI (-1.59 + or - 1.48 vs -0.77 + or - 1.47), BASFI (-1.74 + or - 1.91 vs -0.28 + or - 1.61) and BASMI (-0.51 + or - 1.02 vs -0.21 + or - 0.67); however, differences did not achieve statistical significance. The clinical indices returned to baseline values by 4 weeks after cessation of apremilast. Six apremilast patients (35.3%) vs 3 placebo (15.8%) achieved ASAS20 responses (p=0.25). There were statistically significant decreases in serum RANKL and RANKL:osteoprotegrin ratio and plasma sclerostin but no significant changes in serum DKK-1, bone alkaline phosphatase, TRAP5b, MMP3, osteoprotegrin, or osteocalcin. Although a small pilot study, these results suggest that apremilast may be effective and well tolerated in AS and modulates biomarkers of bone biology. These data support further research of apremilast in axial inflammation.
PMID:22984171 Pathan E et al; Ann Rheum Dis 72 (9): 1475-80 (2013)
EXPL THER Discoid lupus erythematosus (DLE) is a chronic inflammatory disorder mediated by Th1 cells. Apremilast is a novel oral PDE4 enzyme inhibitor capable of blocking leukocyte production of IL-12, IL-23, TNF-a, INF- with subsequent suppression of Th1 and Th17-mediated immune responses, and proven clinical efficacy for psoriasis as well as rheumatoid and psoriatic arthritis. Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) showed a significant (P<0.05) decrease after 85 days of treatment with apremilast 20 mg twice daily in 8 patients with active discoid lupus. The adverse events related to the drug were mild and transient. This is the first open label study to use apremilast as a treatment modality for discoid lupus. Our observations indicate that apremilast may constitute a safe and effective therapeutic option for DLE.
PMID:23134988 De Souza A et al; J Drugs Dermatol 11 (10): 1224-6 (2012)
Treatment with Otezla is associated with an increase in adverse reactions of depression. Before using Otezla in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment with Otezla in such patients. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider. Prescribers should carefully evaluate the risks and benefits of continuing treatment with Otezla if such events occur.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
The safety and effectiveness of Otezla in pediatric patients less than 18 years of age have not been established.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
It is not known whether Otezla or its metabolites are present in human milk; however apremilast was detected in milk of lactating mice. Because many drugs are present in human milk, caution should be exercised when Otezla is administered to a nursing woman.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
For more Drug Warnings (Complete) data for Apremilast (11 total), please visit the HSDB record page.
Apremilast is indicated for the treatment of adults with active psoriatic arthritis and adults with oral ulcers associated with Behcet's Disease. In addition, apremilast is indicated for the treatment of plaque psoriasis, of any severity, in adult patients who are candidates for phototherapy or systemic therapy.
* Psoriatic arthritis :
Otezla, alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs), is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior DMARD therapy.
* Psoriasis:
Otezla is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant to other systemic therapy including cyclosporine, methotrexate or psoralen and ultraviolet-A light (PUVA).
Treatment of Behcet disease
Treatment of chronic idiopathic arthritis (including rheumatoid arthritis , ankylosing spondylitis, psoriatic arthritis and juvenile idiopathic arthritis )
Treatment of psoriasis
Apremilast reduces but does not completely inhibit various inflammatory cytokines such as IL-1, IL-6, IL-8, IL-10 MCP-1, MIP-1, MMP-3, and TNF-, relieving the symptoms of psoriasis and Behcet's disease, which are caused by an increase in these inflammatory mediators. This drug has also been proven to be effective in relieving the pain associated with oral ulcers in Behcet's disease. Apremilast may cause unwanted weight loss and worsen depression, leading to suicidal thoughts or actions. It is advisable to monitor for symptoms of depression and seek medical attention if they occur, especially in patients with pre-existing depression. The need for apremilast should be carefully assessed along with the risk of worsening depression and suicide. If weight loss occurs, the degree of weight loss should be evaluated, and consideration should be made for the possible discontinuation of apremilast.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Phosphodiesterase 4 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 4. (See all compounds classified as Phosphodiesterase 4 Inhibitors.)
L04AA32
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA32 - Apremilast
Absorption
An oral dose of apremilast is well-absorbed and the absolute bioavailability is approximately 73%. Tmax is approximately 2.5 hours and Cmax has been reported to be approximately 584 ng/mL in one pharmacokinetic study. Food intake does not appear to affect apremilast absorption.
Route of Elimination
Only 3% and 7% of an apremilast dose are detected in the urine and feces as unchanged drug, respectively, indicating extensive metabolism and high absorption.
Volume of Distribution
The average apparent volume of distribution (Vd) is about 87 L, suggesting that apremilast is distributed in the extravascular compartment.
Clearance
In healthy patients, the plasma clearance of apremilast is about 10 L/hour.
Human plasma protein binding of apremilast is approximately 68%. Mean apparent volume of distribution (Vd) is 87 L.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
The lacteal excretion of apremilast was evaluated following oral administration of apremilast to lactating CD-1 mice. In this study, female mice approximately 13 days postpartum received a single oral dose of apremilast at 10 mg/kg, administered by oral gavage in a volume of 10 mL/kg. Milk and blood samples from 5 animals per time point were obtained at 1, 6, and 24 hr postdose and apremilast concentrations determined in plasma and milk using LC-MS/MS analysis. The mean apremilast plasma concentrations at 1 and 6 hr post-dose were 984 and 138 ng/mL, while concentrations in milk were 1441 and 186 ng/mL, respectively. The resulting mean milk-to-plasma ratios ranged from 1.46 to 1.62, indicating transfer of apremilast into milk in mice. Plasma and milk concentrations were below the detection limit of 3 ng/mL in the 24-hr samples.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Otezla (Apremilast) p.35 (November 20, 2014). Available from, as of February 26, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf
In monkeys, pregnant animals were administered daily oral doses of apremilast beginning on gestation day 20 through gestation day 50, and a single oral dose on gestation day 100 at dosages of 20, 50, 200, and 1000 mg/kg/day (n = 16/group at the beginning of the study). Maternal and fetal blood was collected at 5 hr postdose on gestation Day 100. In all dosage groups, the fetal-to-maternal plasma concentration ratios were between 0.3 and 0.4, indicating apremilast crossed the placenta in monkeys.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Otezla (Apremilast) p.34-5 (November 20, 2014). Available from, as of February 26, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf
As part of fertility and developmental toxicity study in female CD-1 mice and an embryo-fetal development study in cynomolgus monkeys, the transport of apremilast across the placenta was assessed. In mice, following daily oral administration of apremilast beginning 15 days prior to cohabitation and continuing through Day 15 of presumed gestation at doses of 10, 20, 40, and 80 mg/kg/day, blood was collected from pregnant mice (n = 3/time point) at 0.5, 2, 4, 8, and 24 hr postdose on gestation Day 15. Blood was collected from fetuses) at the time of sacrifice in the 24 hr postdose mice. Maternal plasma apremilast concentrations increased in a less than dose proportional manner. The fetal plasma concentrations at 24 hr were highly variable, with six of the ten litters evaluated being below the limit of quantification (1 ng/mL). In fetal plasma from four of the ten litters evaluated, apremilast was quantified, with concentrations ranging from 14.5 to 2813 ng/mL. The mean fetal-to-maternal plasma concentration ratios ranged from 0.3 to 1.07, indicating apremilast crossed the placenta in mice.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Otezla (Apremilast) p.34 (November 20, 2014). Available from, as of February 26, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf
For more Absorption, Distribution and Excretion (Complete) data for Apremilast (13 total), please visit the HSDB record page.
Apremilast is heavily metabolized by various pathways, which include oxidation, hydrolysis, in addition to conjugation. About 23 metabolites are produced from its metabolism. The CYP3A4 primarily mediates the oxidative metabolism of this drug, with smaller contributions from CYP1A2 and CYP2A6 enzymes. The main metabolite of apremilast, M12, is an inactive glucuronide conjugate form of the O-demethylated drug. Some other major metabolites, M14 and M16, are significantly less active in the inhibition of PDE4 and inflammatory mediators than their parent drug, apremilast. After an oral dose, unchanged apremilast (45%) and the inactive metabolite, O-desmethyl apremilast glucuronide (39%) are found in the plasma. Minor metabolites M7 and M17 are active, but are only present in about 2% or less of apremilast concentrations, and likely not significant contributors to the actions of apremilast.
The plasma clearance of apremilast is about 10 L/hr in healthy subjects, with a terminal elimination half-life of approximately 6-9 hours. Following oral administration of radio-labeled apremilast, about 58% and 39% of the radioactivity is recovered in urine and feces, respectively, with about 3% and 7% of the radioactive dose recovered as apremilast in urine and feces, respectively.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
Following oral administration in humans, apremilast is a major circulating component (45%) followed by inactive metabolite M12 (39%), a glucuronide conjugate of O-demethylated apremilast. It is extensively metabolized in humans with up to 23 metabolites identified in plasma, urine and feces. Apremilast is metabolized by both cytochrome (CYP) oxidative metabolism with subsequent glucuronidation and non-CYP mediated hydrolysis. In vitro, CYP metabolism of apremilast is primarily mediated by CYP3A4, with minor contributions from CYP1A2 and CYP2A6.
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
In /an/ oral study, concentrations of both total radioactivity (e.g., parent compound plus any metabolites) and of parent compound in plasma were greater in females than in males. In males, the total radioactivity AUC values were 25 to 96 times greater than those for parent compound, whereas in females the difference was only 2- to 3-fold, suggesting that metabolism was more extensive in male than in female rats. In the same study following six daily doses, accumulation was indicated by Cmax and AUC values in females, but not in males.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Otezla (Apremilast) p.32 (November 20, 2014). Available from, as of February 26, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf
In a bile-duct cannulated male mouse study following a single 10 mg/kg oral dose of (14)C-apremilast, 54% and 16% of the radioactive dose was excreted via the biliary and urinary routes, suggesting that at least 70% of the radioactive dose was absorbed in mice, indicating apremilast is subject to moderate first pass metabolism. Toxicokinetic evaluation in mice suggests exposure increases dose-proportionally and less than dose-proportionally at doses over 100 mg/kg/day. The studies do not indicate sex-related differences or inversion of apremilast to its R enantiomer in mice.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Otezla (Apremilast) p.32 (November 20, 2014). Available from, as of February 26, 2015: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf
For more Metabolism/Metabolites (Complete) data for Apremilast (6 total), please visit the HSDB record page.
The average elimination half-life of this drug ranges from 6-9 hours.
terminal elimination half-life of approximately 6-9 hours
NIH; DailyMed. Current Medication Information for Otezla (Apremilast) Tablet, Film Coated (Revised: October 2014). Available from, as of February 25, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3acf6751-827d-11e2-9e96-0800200c9a66
The full mechanism of action of this drug is not fully established, however, it is known that apremilast is an inhibitor of phosphodiesterase 4 (PDE4), which mediates the activity of cyclic adenosine monophosphate (cAMP), a second messenger. The inhibition of PDE4 by apremilast leads to increased intracellular cAMP levels. An increase in cAMP results in the suppression of inflammation by decreasing the expression of TNF-, IL-17, IL-23, and other inflammatory mediators. The above inflammatory mediators have been implicated in various psoriatic conditions as well as Behcet's disease, leading to their undesirable inflammatory symptoms such as mouth ulcers, skin lesions, and arthritis. Apremilast administration leads to a cascade which eventually decreases the levels of the above mediators, relieving inflammatory symptoms.
Apremilast is an orally administered phosphodiesterase-4 inhibitor, currently in phase 2 clinical studies of psoriasis and other chronic inflammatory diseases. The inhibitory effects of apremilast on pro-inflammatory responses of human primary peripheral blood mononuclear cells (PBMC), polymorphonuclear cells, natural killer (NK) cells and epidermal keratinocytes were explored in vitro, and in a preclinical model of psoriasis. Apremilast was tested in vitro against endotoxin- and superantigen-stimulated PBMC, bacterial peptide and zymosan-stimulated polymorphonuclear cells, immunonoglobulin and cytokine-stimulated NK cells, and ultraviolet B light-activated keratinocytes. Apremilast was orally administered to beige-severe combined immunodeficient mice, xenotransplanted with normal human skin and triggered with human psoriatic NK cells. Epidermal skin thickness, proliferation index and inflammation markers were analysed. Apremilast inhibited PBMC production of the chemokines CXCL9 and CXCL10, cytokines interferon-gamma and tumour necrosis factor (TNF)-alpha, and interleukins (IL)-2, IL-12 and IL-23. Production of TNF-alpha by NK cells and keratinocytes was also inhibited. In vivo, apremilast significantly reduced epidermal thickness and proliferation, decreased the general histopathological appearance of psoriasiform features and reduced expression of TNF-alpha, human leukocyte antigen-DR and intercellular adhesion molecule-1 in the lesioned skin. Apremilast displayed a broad pattern of anti-inflammatory activity in a variety of cell types and decreased the incidence and severity of a psoriasiform response in vivo. Inhibition of TNF-alpha, IL-12 and IL-23 production, as well as NK and keratinocyte responses by this phosphodiesterase-4 inhibitor suggests a novel approach to the treatment of psoriasis.
PMID:20050849 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2829210 Schafer PH et al; Br J Pharmacol 159 (4): 842-55 (2010)
Psoriasis and psoriatic arthritis are common clinical conditions that negatively impact health-related quality of life and are linked to serious medical comorbidities. Disease mechanisms involve local and systemic chronic inflammatory processes. Available biologic therapies specifically target single inflammatory mediators, such as tumor necrosis factor-a (TNF-a), in the context of a larger inflammatory signaling cascade. To interrupt this pathological cascade earlier in the response or further upstream, and return pro-inflammatory and anti-inflammatory signaling to a homeostatic balance, the use of a phosphodiesterase4 (PDE4) inhibitor has been explored. PDE4 is the major enzyme class responsible for the hydrolysis of cyclic adenosine monophosphate (cAMP), an intracellular second messenger that controls a network of pro-inflammatory and anti-inflammatory mediators. With PDE4 inhibition, and the resulting increases in cAMP levels in immune and non-immune cell types, expression of a network of pro-inflammatory and anti-inflammatory mediators can be modulated. Apremilast is an orally available targeted PDE4 inhibitor that modulates a wide array of inflammatory mediators involved in psoriasis and psoriatic arthritis, including decreases in the expression of inducible nitric oxide synthase, TNF-a, and interleukin (IL)-23 and increases IL-10. In phase II studies of subjects with psoriasis and psoriatic arthritis, apremilast reversed features of the inflammatory pathophysiology in skin and joints and significantly reduces clinical symptoms. The use of an oral targeted PDE4 inhibitor for chronic inflammatory diseases, like psoriasis and psoriatic arthritis, represents a novel treatment approach that does not target any single mediator, but rather focuses on restoring a balance of pro-inflammatory and anti-inflammatory signals.
PMID:22257911 Schafer P; Biochem Pharmacol 83 (12): 1583-90 (2012)
Apremilast, a small-molecule inhibitor of phosphodiesterase 4 (PDE4), works intracellularly to modulate a network of pro-inflammatory and anti-inflammatory mediators. Phosphodiesterase 4 inhibition elevates intracellular cAMP levels, thereby reducing the inflammatory response by modulating the expression of tumor necrosis factor-alpha (TNF-a), interleukin-23 (IL-23), IL-17 and IL-10.
Health Canada; Product Monograph for Otezla (Apremilast), Drug Identification Number (DIN): 02434318 p.13 (Date of Preparation: November 12, 2014). Available from, as of February 25, 2015: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng