


1. 5,10-methylene-5,6,7,8-tetrahydrofolate
2. 5,10-methylenetetrahydrofolate
3. 5,10-methylenetetrahydrofolate Monohydrochloride, (l-glu)-isomer
4. 5,10-methylenetetrahydrofolate, (d-glu)-isomer
5. 5,10-methylenetetrahydrofolate, (l-glu)-(s)-isomer
6. 5,10-methylenetetrahydrofolic Acid
7. Ch2h4fol
8. Ch2h4folate
9. N5,n10-methylene-5,6,7,8-tetrahydrofolic Acid
10. Tetrahydromethylenefolate
1. Arfolitixorin
2. 31690-11-6
3. Modufolin
4. 6r-mthf
5. Methylenetetrahydrofolic Acid, L-(+)-
6. Arfolitixorin [usan]
7. (6r)-5,10-methylenetetrahydrofolic Acid
8. Chebi:1989
9. Z8r4a37v9q
10. Iso-901
11. L-(+)-methylenetetrahydrofolic Acid
12. 5,10-methylene-thf
13. 5,10-methylene-(6r)-tetrahydrofolic Acid
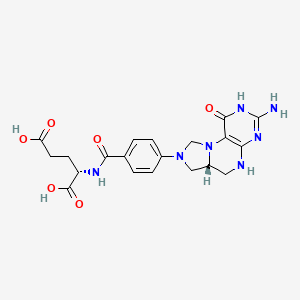
14. (2s)-2-[[4-[(6ar)-3-amino-1-oxo-4,5,6,6a,7,9-hexahydroimidazo[1,5-f]pteridin-8-yl]benzoyl]amino]pentanedioic Acid
15. 3432-99-3
16. 5,10-methylenetetrahydrofolate
17. N-({4-[(6ar)-3-amino-1-oxo-1,2,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9h)-yl]phenyl}carbonyl)-l-glutamic Acid
18. N-{4-[(6ar)-3-amino-1-oxo-1,2,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9h)-yl]benzoyl}-l-glutamic Acid
19. Unii-z8r4a37v9q
20. Arfolitixorin [inn]
21. Arfolitixorin (usan/inn)
22. Schembl186747
23. Arfolitixorin [who-dd]
24. Chembl1234270
25. Dtxsid60185584
26. Bcp09003
27. Zinc4228243
28. (6r)-5,10-mthf
29. Who 10689
30. Db12676
31. 5,10-methylene,5,6,7,8-tetrahydrofolate
32. D11447
33. D-n5,n10-methylene-l-tetrahydrofolic Acid
34. Q26995704
35. (6r,s)-5,10-methylene-5,6,7,8-tetrahydrofolic Acid
36. [6r]-5,10-methylenetetrahydrofolate ([6r]-5,10- Mthf)
37. (6r)-5,10-methylene-5,6,7,8-tetrahydrofolic Acid
38. (2s)-2-[[4-[(6ar)-3-amino-1-oxo-2,5,6,6a,7,9-hexahydroimidazo[1,5-f]pteridin-8-yl]benzoyl]amino]pentanedioic Acid
39. (s)-2-(4-((r)-3-amino-1-oxo-5,6,6a,7-tetrahydroimidazo[1,5-f]pteridin-8(1h,4h,9h)-yl)benzamido)pentanedioic Acid
40. (s)-2-(4-((r)-3-amino-1-oxo-5,6,6a,7-tetrahydroimidazo[1,5-f]pteridin-8(1h,4h,9h)-yl)benzamido)pentanedioicacid
41. L-glutamic Acid, N-(4-((6ar)-3-amino-1,2,5,6,6a,7-hexahydro-1-oxoimidazo(1,5-f)pteridin-8(9h)-yl)benzoyl)-
42. N-({4-[(6ar)-3-amino-1-oxo-1,4,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9h)-yl]phenyl}carbonyl)-l-glutamic Acid
43. N-(4-((6ar)-3-amino-1-oxo-1,2,5,6,6a,7-hexahydroimidazo(1,5-f)pteridin-8(9h)- Yl)benzoyl)-l-glutamic Acid
| Molecular Weight | 457.4 g/mol |
|---|---|
| Molecular Formula | C20H23N7O6 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 457.17098148 g/mol |
| Monoisotopic Mass | 457.17098148 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 911 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of colorectal cancer
