

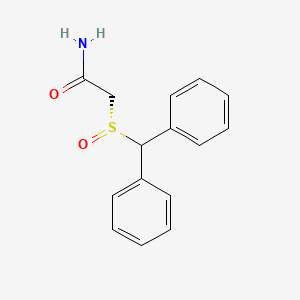

1. 2 Benzhydrylsulfinylacetamide
2. 2-((diphenylmethyl)sulfinyl)acetamide
3. 2-((r)-(diphenylmethyl)sulfinyl)acetamide
4. 2-(benzhydrylsulfinyl)acetamide
5. 2-benzhydrylsulfinylacetamide
6. Alertec
7. Benzhydrylsulfinylacetamide
8. Crl 40476
9. Crl-40476
10. Modafinil
11. Modiodal
12. Nuvigil
13. Provigil
14. R Modafinil
15. R-modafinil
16. Sparlon
1. 112111-43-0
2. Nuvigil
3. (r)-modafinil
4. (-)-modafinil
5. (r)-(-)-modafinil
6. Cep-10953
7. Crl 40982
8. Modafinil, (r)-
9. R-(-)-modafinil
10. 2-[(r)-(diphenylmethyl)sulfinyl]acetamide
11. (-)-(r)-modafinil
12. Cep 10953
13. Cep-10952
14. Crl-40982
15. V63xwa605i
16. Chembl1201192
17. Chebi:77590
18. Nsc-751850
19. Nsc-758711
20. (-)-2-((r)-(diphenylmethyl)sulfinyl)acetamide
21. Acetamide, 2-((diphenylmethyl)sulfinyl)-, (-)-
22. Armodafinil [inn]
23. (-)-2-[(r)-(diphenylmethyl)sulfinyl]acetamide
24. Armodafinil [usan:inn]
25. Armodafinilo
26. Armodafinilum
27. Unii-v63xwa605i
28. L-modafinil
29. (-) Modafinil
30. Nuvigil (tn)
31. Armodafinil (usan/inn)
32. Armodafinil [usan]
33. Armodafinil [vandf]
34. Armodafinil [mart.]
35. Schembl34489
36. Armodafinil [usp-rs]
37. Armodafinil [who-dd]
38. Zinc6156
39. Armodafinil, >=98% (hplc)
40. Armodafinil [orange Book]
41. Dtxsid90920667
42. 2-[(r)-benzhydrylsulfinyl]acetamide
43. Bdbm50336892
44. Akos030211019
45. At22562
46. Ccg-230228
47. Cs-0665
48. Db06413
49. Nsc 751850
50. Nsc 758711
51. 2-[(r)-diphenylmethanesulfinyl]acetamide
52. Hy-15201
53. (-)-2r-[(diphenylmethyl)sulfinyl]acetamide
54. S4645
55. D03215
56. Q418913
57. Acetamide, 2-((r)-(diphenylmethyl)sulfinyl)-
| Molecular Weight | 273.4 g/mol |
|---|---|
| Molecular Formula | C15H15NO2S |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 273.08234989 g/mol |
| Monoisotopic Mass | 273.08234989 g/mol |
| Topological Polar Surface Area | 79.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Armodafinil |
| PubMed Health | Armodafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | NUVIGIL (armodafinil) is a wakefulness-promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S-enantiomers. The chemical name for armodafinil is 2-[(R)-(diphenylmethyl)sulfinyl]acetam... |
| Active Ingredient | Armodafinil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 2500mg; 200mg; 250mg; 100mg; 50mg; 150mg |
| Market Status | Tentative Approval; Prescription |
| Company | Watson Labs; Mylan Pharms; Teva Pharms Usa; Lupin |
| 2 of 4 | |
|---|---|
| Drug Name | Nuvigil |
| PubMed Health | Armodafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | NUVIGIL (armodafinil) is a wakefulness-promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S-enantiomers. The chemical name for armodafinil is 2-[(R)-(diphenylmethyl)sulfinyl]acetam... |
| Active Ingredient | Armodafinil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 250mg; 150mg; 50mg |
| Market Status | Prescription |
| Company | Cephalon |
| 3 of 4 | |
|---|---|
| Drug Name | Armodafinil |
| PubMed Health | Armodafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | NUVIGIL (armodafinil) is a wakefulness-promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S-enantiomers. The chemical name for armodafinil is 2-[(R)-(diphenylmethyl)sulfinyl]acetam... |
| Active Ingredient | Armodafinil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 2500mg; 200mg; 250mg; 100mg; 50mg; 150mg |
| Market Status | Tentative Approval; Prescription |
| Company | Watson Labs; Mylan Pharms; Teva Pharms Usa; Lupin |
| 4 of 4 | |
|---|---|
| Drug Name | Nuvigil |
| PubMed Health | Armodafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | NUVIGIL (armodafinil) is a wakefulness-promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S-enantiomers. The chemical name for armodafinil is 2-[(R)-(diphenylmethyl)sulfinyl]acetam... |
| Active Ingredient | Armodafinil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 250mg; 150mg; 50mg |
| Market Status | Prescription |
| Company | Cephalon |
Investigated for use/treatment in sleep disorders, obstructive sleep apnea, schizophrenia and schizoaffective disorders, depression, and bipolar disorders.
FDA Label
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Wakefulness-Promoting Agents
A specific category of drugs that prevent sleepiness by specifically targeting sleep-mechanisms in the brain. They are used to treat DISORDERS OF EXCESSIVE SOMNOLENCE such as NARCOLEPSY. Note that this drug category does not include broadly-acting central nervous system stimulants such as AMPHETAMINES. (See all compounds classified as Wakefulness-Promoting Agents.)
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BA - Centrally acting sympathomimetics
N06BA13 - Armodafinil
Absorption
Tmax is 2 hours when fasted and can be delayed approximately 2-4 hours by food, potentially affecting the onset of action.
Volume of Distribution
Apparent volume of distribution: 42L.
Clearance
The oral clearance of armodafinil is approximately 33 mL/min.
In vitro and in vivo data show that armodafinil undergoes hydrolytic deamidation, S-oxidation, and aromatic ring hydroxylation, with subsequent glucuronide conjugation of the hydroxylated products. Amide hydrolysis is the single most prominent metabolic pathway, with sulfone formation by cytochrome P450 (CYP) 3A4/5 being next in importance. The other oxidative products are formed too slowly in vitro to enable identification of the enzyme(s) responsible. Only two metabolites reach appreciable concentrations in plasma (i.e., R-modafinil acid and modafinil sulfone). Data specific to armodafinil disposition are not available.
Terminal half-life is approximately 15 hours.
Nuvigil (armodafinil) is a single-isomer of modafini. The exact mechanism of action is unknown. Armodafinil belongs to a class of drugs known as eugeroics, which are stimulants that provide long-lasting mental arousal. Pharmacologically, armodafinil does not bind to or inhibit several receptors and enzymes potentially relevant for sleep/wake regulation. Armodafinil is not a direct- or indirect-acting dopamine receptor agonist. However, in vitro, both armodafinil and modafinil bind to the dopamine transporter and inhibit dopamine reuptake. [Medilexicon]