


1. Abl001
2. Asciminib Hydrochloride
1. Abl-001
2. 1492952-76-7
3. Abl001
4. Asciminib Free Base
5. Abl001-nx
6. Nvp-abl001
7. Asciminib [usan]
8. Scemblix
9. Example 9
10. L1f3r18w77
11. 1492952-76-7 (free Base)
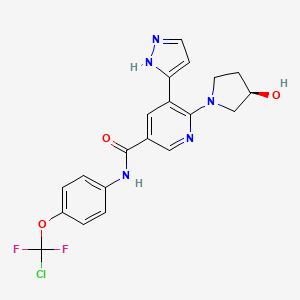
12. (r)-n-(4-(chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1h-pyrazol-5-yl)nicotinamide
13. 3-pyridinecarboxamide, N-(4-(chlorodifluoromethoxy)phenyl)-6-((3r)-3-hydroxy-1-pyrrolidinyl)-5-(1h-pyrazol-3-yl)-
14. N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3r)-3-hydroxypyrrolidin-1-yl]-5-(1h-pyrazol-5-yl)pyridine-3-carboxamide
15. 3-pyridinecarboxamide, N-[4-(chlorodifluoromethoxy)phenyl]-6-[(3r)-3-hydroxy-1-pyrrolidinyl]-5-(1h-pyrazol-3-yl)-
16. Asciminib [inn]
17. Asciminib (abl001)
18. Asciminib (usan/inn)
19. Asciminib [who-dd]
20. Unii-l1f3r18w77
21. Gtpl8962
22. Chembl4208229
23. Schembl15388306
24. Tqp0925
25. Ex-a3030
26. Bdbm50459091
27. Nsc789925
28. S8555
29. Zinc150275965
30. At30330
31. Ccg-269232
32. Compound 1 [pmid: 30137981]
33. Cs-7655
34. Db12597
35. Nsc-789925
36. (r)-n- (4-(chlorodifluoromethoxy)phenyl)- 6-(3- Hydroxypyrrolidin-1- Yl)-5- (1h-pyrazol- 5-yl)nicotinamide
37. Ba166957
38. Bs-15538
39. Hy-104010
40. D11403
41. A910986
42. Q27074535
43. (r)-n-(4-(chloro Difluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1h-pyrazol-5-yl)nicotinamide
44. (r)-n-(4-(chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1h-pyrazol-3-yl)nicotinamide
45. Ay7
46. N-(4-(chlorodifluoromethoxy)phenyl)-6-((3r)-3- Hydroxypyrrolidin-1-yl)-5-(1h-pyrazol-3-yl)pyridine- 3-carboxamide
| Molecular Weight | 449.8 g/mol |
|---|---|
| Molecular Formula | C20H18ClF2N5O3 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 449.1066235 g/mol |
| Monoisotopic Mass | 449.1066235 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 626 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Asciminib is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase who have been previously treated with 2 tyrosine kinase inhibitors. It is also indicated in the treatment of Ph+ CML in adult patients with the T315I mutation.
Scemblix is indicated for the treatment of adult patients with Philadelphia chromosome positive chronic myeloid leukaemia in chronic phase (Ph+ CML CP) previously treated with two or more tyrosine kinase inhibitors (see section 5. 1).
Asciminib exerts its therapeutic activity by inhibiting an oncogenic protein responsible for the proliferation of CML. It may be administered orally once or twice a day depending on the condition being treated. By increasing the total daily dose 5-fold as compared to standard therapy (80mg daily vs. 400mg daily), it can be used to treat Ph+ CML with the T315I mutation, a typically treatment-resistant variant of the disease. As with many other chemotherapeutic agents, asciminib treatment can result in various forms of myelosuppression, including thrombocytopenia and neutropenia. Patients should receive frequent laboratory monitoring throughout therapy and dose adjustments may be required based on the severity of observed effects. Patients may also experience pancreatic and/or cardiovascular toxicity, both of which require frequent monitoring and may require dose adjustments as per prescribing information.
L01EA06
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EA - Bcr-abl tyrosine kinase inhibitors
L01EA06 - Asciminib
Absorption
The median Tmax of asciminib following oral administration is 2.5 hours. At a dose of 80mg once daily, the steady-state Cmax and AUCtau were 1781 ng/mL and 15112 ng.h/mL, respectively. At a dose of 40mg twice daily, the steady-state Cmax and AUCtau were 793 ng/mL and 5262 ng.h/mL, respectively. At a dose of 200mg twice daily (for treatment of T315I mutants), the steady-state Cmax and AUCtau were 5642 ng/mL and 37547 ng.h/mL, respectively. As compared to the fasted state, the co-administration of asciminib with a high-fat meal decreased the AUC and Cmax by 62% and 68%, respectively, and its co-administration with a low-fat meal decreased the AUC and Cmax by 30% and 35%, respectively.
Route of Elimination
Asciminib is eliminated via biliary secretion facilitated by breast cancer-resistant protein (BCRP) transporters. Following oral administration, approximately 80% and 11% of an asciminib dose was recovered in the feces and urine, respectively. Unchanged parent drug accounted for 57% of drug material recovered in the feces and 2.5% in the urine.
Volume of Distribution
At steady-state, the apparent volume of distribution of asciminib is 151 L.
Clearance
The total apparent clearance of asciminib is 6.7 L/h at a total daily dose of 80mg and 4.1 L/h at a dose of 200mg twice daily.
Asciminib is negligibly metabolized, with unchanged parent drug comprising the main drug component in plasma (~93%) and following excretion (~57% in feces). The main circulating metabolites are M30.5, M44, and M29.5, accounting for approximately 5%, 2%, and 0.4% of the total administered dose, respectively. The oxidative metabolism of asciminib is mediated by CYP3A4, and the glucuronidation of asciminib is mediated by UGT2B7 and UGT2B17.
The terminal elimination half-life asciminib is 5.5 hours when administered at 40mg twice daily and 9.0 hours when administered at 200mg twice daily.
In most patients with chronic myeloid leukemia (CML), progression of the disease is driven primarily by a translocation of the Philadelphia chromosome that creates an oncogenic fusion gene, _BCR-ABL1_, between the _BCR_ and _ABL1_ genes. This fusion gene produces a resultant fusion protein, BCR-ABL1, which exhibits elevated tyrosine kinase and transforming activities that contribute to CML proliferation. Asciminib is an allosteric inhibitor of the BCR-ABL1 tyrosine kinase. It binds to the myristoyl pocket of the ABL1 portion of the fusion protein and locks it into an inactive conformation, preventing its oncogenic activity.