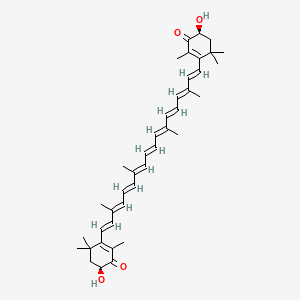

1. Astaxanthine
2. E-astaxanthin
1. 472-61-7
2. Ovoester
3. All-trans-astaxanthin
4. Astareal
5. 3,3'-dihydroxy-beta,beta-carotene-4,4'-dione
6. Astaxanthine
7. Bioastin
8. (3s,3's)-astaxanthin
9. Trans-astaxanthin
10. Astaxanthin, (3s,3's)-
11. (3s,3's)-all-trans-astaxanthin
12. 8xpw32pr7i
13. 3,3'-dihydroxy-beta-carotene-4,4'-dione
14. (3s,3's)-3,3'-dihydroxy-beta,beta-carotene-4,4'-dione
15. Chebi:40968
16. Nsc-635689
17. (6s,6's)-3,3'-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(6-hydroxy-2,4,4-trimethylcyclohex-2-enone)
18. .beta.,.beta.-carotene-4,4'-dione, 3,3'-dihydroxy-, (3s,3's)-
19. Algae Haematococcus Pluvialis
20. Natupink
21. Astaxin
22. Carophyll Pink
23. Lucantin Pink
24. Bioastin Oleoresin
25. (6s)-6-hydroxy-3-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-[(4s)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone
26. Astaxanthin (6ci)
27. Astaxanthin, All-trans-
28. Unii-8xpw32pr7i
29. Astaxantin
30. Ccris 7118
31. Hsdb 7468
32. (6s)-6-hydroxy-3-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-[(4s)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohexen-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethylcyclohex-2-en-1
33. Einecs 207-451-4
34. Nsc 635689
35. 3s,3's-astaxanthin
36. Astaxanthin, All-trans-, (3s,3's)-
37. Astaxanthin, 5% Active
38. Astaxanthin [mi]
39. B-carotene-4,4'-dione
40. Astaxanthin [hsdb]
41. Astaxanthin [inci]
42. Coriolus Versicolor Extract
43. Astaxanthin [vandf]
44. Astaxanthin [mart.]
45. Schembl20047
46. Astaxanthin [usp-rs]
47. Astaxanthin [who-dd]
48. E161j
49. Chembl1255871
50. Astaxanthin, >=98% (hplc)
51. E 161j
52. Dtxsid00893777
53. All-trans-(3s,3's)-astaxanthin
54. 3,3'-dihydroxy-beta,beta-carotene-4,4'-dione, (3s,3's)-
55. Hms3885c08
56. Bcp05821
57. Hy-b2163
58. Lmpr01070263
59. Mfcd00672621
60. S3834
61. Akos015841055
62. Akos015895756
63. Zinc100042059
64. Ac-8760
65. Bcp9000329
66. Ccg-270185
67. Db06543
68. 3,3'-dihydroxy-ss-carotene-4,4'-dione
69. All-trans-astaxanthin, Analytical Standard
70. As-14095
71. Astaxanthin 10 Microg/ml In Acetonitrile
72. Cs-0020413
73. C08580
74. M01303
75. 3,3'-dihydroxy-4,4'-diketo-beta,beta-carotene
76. 472a617
77. A827177
78. Q413740
79. Q-200655
80. 3,3'-dihydroxy-4,4'-diketo-.beta.-carotene
81. All-trans-3,3'-dihydroxy-b-carotene-4,4'-dione (8ci)
82. .beta.-carotene-4,4'-dione, 3,3'-dihydroxy-, All-trans-
83. 3-(4,6-dimethyl-2-oxo-2h-pyrimidin-1-yl)-propionicacid
84. All-trans-3,3'-dihydroxy-beta-carotene-4,4'-dione (8ci)
85. Astaxanthin (constituent Of Astaxanthin Esters) [dsc]
86. 3,3'-dihydroxy-.beta.,.beta.-carotene-4,4'-dione, (3s,3's)-
87. (3s,3's)-3,3'-dihydroxy-.beta.,.beta.-carotene-4,4'-dione
88. Astaxanthin. Short Expiry Date Due To Chemical Nature Of Component(s)
89. 3,3'-dihydroxy-beta,beta-carotene-4,4'-dione;(s)-6-hydroxy-3-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-((s)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohex-1-enyl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl)-2,4,4-trimethylcyclohex-2-enone;
| Molecular Weight | 596.8 g/mol |
|---|---|
| Molecular Formula | C40H52O4 |
| XLogP3 | 10.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 10 |
| Exact Mass | 596.38656014 g/mol |
| Monoisotopic Mass | 596.38656014 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1340 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 9 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the current study, it was hypothesized that oral Cardax /disodium disuccinate astaxanthin/ administration would inhibit oxidative damage of multiple relevant biological targets in a representative, well-characterized murine peritoneal inflammation model. A previously developed mass spectrometry-based (LC/ESI/MS/MS) approach was used to interrogate multiple distinct pathways of oxidation in a black mouse (C57/BL6) model system. In vivo markers of oxidant stress from peritoneal lavage samples (supernatants) were evaluated in mice on day eight (8) after treatment with either Cardax or vehicle (lipophilic emulsion without drug) orally by gavage at 500 mg/kg once per day for seven (7) days at five (5) time points: (1) baseline prior to treatment (t=0); (2) 16 h following intraperitoneal (i.p.) injection with thioglycollate to elicit a neutrophilic infiltrate; (3) 4 h following i.p. injection of yeast cell wall (zymosan; t=16 h/4 h thioglycollate+zymosan); (4) 72 h following i.p. injection with thioglycollate to elicit monocyte/macrophage infiltration; and (5) 72 h/4 h thioglycollate+zymosan. A statistically significant sparing effect on the arachidonic acid (AA) and linoleic acid (LA) substrates was observed at time points two and five. When normalized to the concentration of the oxidative substrates, statistically significant reductions of 8-isoprostane-F(2alpha) (8-iso-F(2alpha)) at time point three (maximal neutrophil recruitment/activation), and 5-HETE, 5-oxo-EET, 11-HETE, 9-HODE, and PGF(2alpha) at time point five (maximal monocyte/macrophage recruitment/activation) were observed. Subsequently, the direct interaction of the optically inactive stereoisomer of Cardax (meso-dAST) with human 5-lipoxygenase (5-LOX) was evaluated in vitro with circular dichroism (CD) and electronic absorption (UV/Vis) spectroscopy, and subsequent molecular docking calculations were made using mammalian 15-LOX as a surrogate (for which XRC data has been reported). The results suggested that the meso-compound was capable of interaction with, and binding to, the solvent-exposed surface of the enzyme. These preliminary studies provide the foundation for more detailed evaluation of the therapeutic effects of this compound on the 5-LOX enzyme, important in chronic diseases such as atherosclerosis, asthma, and prostate cancer in humans. /Disodium disuccinate astaxanthin/
PMID:16466747 Lockwood SF et al; Life Sci 79 (2): 162-74 (2006)
The composition of atherosclerotic plaques, not just macroscopical lesion size, has been implicated in their susceptibility to rupture and the risk of thrombus formation. By focusing on the quality of lipids, macrophages, apoptosis, collagen, metalloproteinase expression and plaque integrity, we evaluated the possible anti-atherosclerotic effect of the antioxidants alpha-tocopherol and astaxanthin in Watanabe heritable hyperlipidemic (WHHL) rabbits. Thirty-one WHHL rabbits were divided into three groups and were fed a standard diet, as controls (N =10), or a standard diet with the addition of 500 mg alpha-tocopherol per kg feed (N =11) or 100 mg astaxanthin per kg feed (N =10) for 24 weeks. We found that both antioxidants, particularly astaxanthin, significantly decreased macrophage infiltration in the plaques although they did not affect lipid accumulation. All lesions in the astaxanthin-treated rabbits were classified as early plaques according to the distribution of collagen and smooth muscle cells. Both antioxidants also improved plaque stability and significantly diminished apoptosis, which mainly occurred in macrophages, matrix metalloproteinase three expressions and plaque ruptures. Although neither antioxidant altered the positive correlations between the lesion size and lipid accumulation, the lesion size and apoptosis were only positively correlated in the control group. Astaxanthin and alpha-tocopherol may improve plaque stability by decreasing macrophage infiltration and apoptosis in this atherosclerotic setting. Apoptosis reduction by alpha-tocopherol and astaxanthin may be a new anti-atherogenic property of these antioxidants.
PMID:15522274 Li W et al; J Mol Cell Cardiol 37 (5): 969-78 (2004)
Exptl Ther: Astaxanthin, a carotenoid without vitamin A activity, may exert antitumor activity through the enhancement of immune responses. Here, we determined the effects of dietary astaxanthin on tumor growth and tumor immunity against transplantable methylcholanthrene-induced fibrosarcoma (Meth-A tumor) cells. These tumor cells express a tumor antigen that induces T cell-mediated immune responses in syngenic mice. BALB/c mice were fed astaxanthin (0.02%, 40 micrograms/kg body wt/day in a beadlet form) mixed in a chemically defined diet starting zero, one, and three weeks before subcutaneous inoculation with tumor cells (3 x 10(5) cells, 2 times the minimal tumorigenic dose). Three weeks after inoculation, tumor size and weight were determined. We also determined cytotoxic T lymphocyte (CTL) activity and interferon-gamma (IFN-gamma) production by tumor-draining lymph node (TDLN) and spleen cells by restimulating cells with Meth-A tumor cells in culture. The astaxanthin-fed mice had significantly lower tumor size and weight than controls when supplementation was started one and three weeks before tumor inoculation. This antitumor activity was paralleled with higher CTL activity and IFN-gamma production by TDLN and spleen cells in the astaxanthin-fed mice. CTL activity by TDLN cells was highest in mice fed astaxanthin for three weeks before inoculation. When the astaxanthin-supplemented diet was started at the same time as tumor inoculation, none of these parameters were altered by dietary astaxanthin, except IFN-gamma production by spleen cells. Total serum astaxanthin concentrations were approximately 1.2 mumol/l when mice were fed astaxanthin (0.02%) for four weeks and appeared to increase in correlation with the length of astaxanthin supplementation. Our results indicate that dietary astaxanthin suppressed Meth-A tumor cell growth and stimulated immunity against Meth-A tumor antigen.
PMID:10798217 Jyonouchi H et al; Nutr Cancer 36 (1): 59-65 (2000)
Exptl Ther: In the current study, the improved oral bioavailability of a synthetic astaxanthin derivative (Cardax; disodium disuccinate astaxanthin) was utilized to evaluate its potential effects as a cardioprotective agent after 7-day subchronic oral administration as a feed supplement to Sprague-Dawley rats. Animals received one of two concentrations of Cardax in feed (0.1 and 0.4%; approximately 125 and 500 mg/kg/day, respectively) or control feed without drug for 7 days prior to the infarct study carried out on day 8. Thirty minutes of occlusion of the left anterior descending (LAD) coronary artery was followed by 2 hr of reperfusion prior to sacrifice, a regimen which resulted in a mean infarct size (IS) as a percentage (%) of the area at risk (AAR; IS/AAR,%) of 61 + or - 1.8%. The AAR was quantified by Patent blue dye injection, and IS was determined by triphenyltetrazolium chloride (TTC) staining. Cardax at 0.1 and 0.4% in feed for 7 days resulted in a significant mean reduction in IS/AAR,% to 45 + or - 2.0% (26% salvage) and 39 + or - 1.5% (36% salvage), respectively. Myocardial levels of free astaxanthin achieved after 7-day supplementation at each of the two concentrations (400 + or - 65 nM and 1634 + or - 90 nM, respectively) demonstrated excellent solid-tissue target organ loading after oral supplementation. Parallel trends in reduction of plasma levels of multiple lipid peroxidation products with disodium disuccinate astaxanthin supplementation were observed, consistent with the documented in vitro antioxidant mechanism of action. These results extend the potential utility of this compound for cardioprotection to the elective human cardiovascular patient population, for which 7-day oral pre-treatment (as with statins) provides significant reductions in induced periprocedural infarct size. /Disodium disuccinate astaxanthin/
PMID:16444582 Gross GJ et al; : Mol Cell Biochem 283 (1-2): 23-30 (2006)
For more Therapeutic Uses (Complete) data for ASTAXANTHINE (7 total), please visit the HSDB record page.
Investigated for use/treatment in eye disorders/infections, cancer/tumors (unspecified), and asthma.
Apparent astaxanthin (3,3'-dihydroxy-beta,beta-carotene-4,4'-dione) digestibility coefficients (ADC) and carotenoid compositions of the muscle, liver, whole kidney and plasma were compared in Atlantic salmon (Salmo salar) and Atlantic halibut (Hippoglossus hippoglossus) fed a diet supplemented with 66 mg astaxanthin kg(-1) dry matter for 112 days. The astaxanthin source consisted of 75% all-E-, 3% 9Z- and 22% 13Z-astaxanthin, of (3R,3'R)-, (3R,3'S; meso)-, and (3S,3'S)-astaxanthin in a 1:2:1 ratio. The ADC of astaxanthin was significantly higher in Atlantic halibut than in Atlantic salmon after 56 and 112 days of feeding (P < 0.05). The ADC of all-E-astaxanthin was significantly higher than ADC of 9Z-astaxanthin (P < 0.05). Considerably more carotenoids were present in all plasma and tissue samples of salmon than in halibut. Retention of astaxanthin in salmon muscle was 3.9% in salmon and 0 in halibut. All-E-astaxanthin accumulated selectively in the muscle of salmon, and in plasma of salmon and halibut compared with diet. 13Z-astaxanthin accumulated selectively in liver and whole kidney of salmon and halibut, when compared with plasma. A reductive pathway for astaxanthin metabolism in halibut similar to that of salmon was shown by the presence of 3',4'-cis and trans glycolic isomers of idoxanthin (3,3',4'-trihydroxy-beta,beta-carotene-4'-one) in plasma, liver and whole kidney. In conclusion, the higher ADC of astaxanthin in halibut than Atlantic salmon may be explained by lower feed intake in halibut, and the lower retention of astaxanthin by a higher capacity to transform astaxanthin metabolically.
PMID:11126773 Bjerkeng B, Berge GM; Comp Biochem Physiol B Biochem Mol Biol 127 (3): 423-32 (2000)
The present studies were performed to investigate the metabolism of astaxanthin (Ax) in Atlantic salmon, especially in the liver of salmon. The investigations were undertaken in vivo salmon that were fed a diet containing 60 ppm 15, 15' (14)C-labelled Ax prior to sacrifice. The samples of blood, bile, liver, gastrointestinal tract and contents, muscle, skin, remaining carcass and feces were taken for scintillation counting. The highest radioactivity (71.36%) of (14)C-labelled Ax was found in the gastrointestinal contents and feces, 7.13% in the bile and 10.68% in the samples of liver, muscle, and skin at the end of the experiments. The metabolites of (14)C-labelled Ax were extracted from the bile of the salmon and analyzed using thin-layer chromatography (TLC) and high performance liquid chromatography (HPLC). Predominant (14)C-labelled Ax and its cis-isomers were found and no conjugation of (14)C-labelled Ax was observed. These results indicate that (14)C-labelled Ax was not conjugated into larger colorless compound in Atlantic salmon liver.
PMID:15039990 Xu Y, Ding Z; J Exp Zoolog A Comp Exp Biol 301 (4):317-23 (2004)
One force-fed meal containing labelled (14)C-astaxanthin and (3)H-canthaxanthin or (3)H-zeaxanthin was given to eight mature female rainbow trout. Ninety-six hours after the test meal ingestion, trout were killed and liver, skin, muscle and ovaries were dissected out. Astaxanthin accumulated slightly more in muscle than canthaxanthin but in all tissues astaxanthin and canthaxanthin were very significantly more concentrated than zeaxanthin. (3)H-zeaxanthin metabolites were found only in the liver, whereas (14)C-phoenicoxanthin was the only metabolic pigment from (14)C-astaxanthin detected and was found in all investigated tissues. (3)H-astaxanthin was found in the liver of all trout indicating that (3)H-canthaxanthin and (3)H-zeaxanthin were astaxanthin precursors, and that salmonids probably possess carotenoid oxidative pathways unknown until now. Labelled retinol1 and retinol2 were detected only in the liver and (3)H-zeaxanthin was largely the predominant precursor of these two vitamin A forms.
PMID:1526135 Guillou A et al; 1: Comp Biochem Physiol B 102 (1): 61-5 (1992)
The effects of feed intake, growth rate and temperature (8 and 12 degrees C) on apparent digestibility coefficients (ADC), blood uptake of individual astaxanthin E/Z isomers and metabolism of astaxanthin (3,3'-dihydroxy-beta,beta-carotene-4,4'-dione) were determined in Atlantic salmon. Accumulation of idoxanthin (3,4,3'-trihydroxy-beta,beta-carotene-4-one) in plasma was used to indicate metabolic transformation of astaxanthin.
PMID:16242366 Ytrestoyl T et al; Comp Biochem Physiol B Biochem Mol Biol 142 (4): 445-55 (2005)