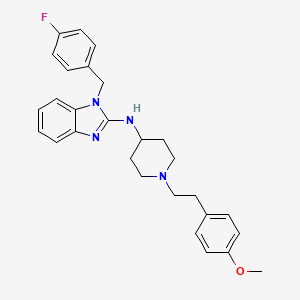

1. Alermizol
2. Alonga, Astemizol
3. Astemina
4. Astemizol Alonga
5. Astemizol Ratiopharm
6. Astesen
7. Emdar
8. Esmacen
9. Fustermizol
10. Hismanal
11. Histaminos
12. Hubermizol
13. Laridal
14. Paralergin
15. R 43 512
16. R-43-512
17. R43512
18. Ratiopharm, Astemizol
19. Retolen
20. Rifedot
21. Rimbol
22. Romadin
23. Simprox
24. Urdrim
1. 68844-77-9
2. Hismanal
3. Histaminos
4. Paralergin
5. Laridal
6. Retolen
7. Astemison
8. Histamen
9. Kelp
10. Astemizol
11. Astemizolum
12. 1-[(4-fluorophenyl)methyl]-n-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1h-benzimidazol-2-amine
13. 1-(p-fluorobenzyl)-2-((1-(p-methoxyphenethyl)-4-piperidyl)amino)benzimidazole
14. Alermizol
15. 1-[(4-fluorophenyl)methyl]-n-[1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl]benzimidazol-2-amine
16. R 42512
17. Gnf-pf-2461
18. 1-(p-fluorobenzyl)-2-((1-(2-(p-methoxyphenyl)ethyl)piperid-4-yl)amino)benzimidazole
19. Astemisan
20. Nsc-329963
21. Nsc-759570
22. Mls000028667
23. Chebi:2896
24. Chembl296419
25. Hestazol
26. Histazol
27. Metodih
28. Wareezol
29. Novo-mastizol A
30. R 43,512
31. 1h-benzimidazol-2-amine, 1-((4-fluorophenyl)methyl)-n-(1-(2-(4-methoxyphenyl)ethyl)-4-piperidinyl)-
32. Astemizol [german]
33. Ncgc00016913-08
34. Metodik
35. Smr000058911
36. Waruzol
37. Cas-68844-77-9
38. Dsstox_cid_110
39. 7hu6337315
40. 1-(4-fluorobenzyl)-2-(1-[4-methoxyphenethyl]piperidin-4-yl)aminobenzimidazole
41. Benzimidazole, 1-(p-fluorobenzyl)-2-((1-(2-(p-methoxyphenyl)ethyl)piperid-4-yl)amino)-
42. Dsstox_rid_75373
43. Astemizol [inn-spanish]
44. Astemizolum [inn-latin]
45. Dsstox_gsid_20110
46. R 43512
47. 1-[(4-fluorophenyl)methyl]-n-{1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl}-1h-benzimidazol-2-amine
48. 1h-benzimidazol-2-amine, 1-[(4-fluorophenyl)methyl]-n-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-
49. 1-((4-fluorophenyl)methyl)-n-(1-(2-(4-methoxyphenyl)ethyl)-4-piperidinyl)- 1h-benzimidazol-2-amine
50. Novo-nastizol A
51. [3h]astemizole
52. 1-(4-fluorobenzyl)-2-(1-(4-methoxyphenethyl)piperidin-4-yl)aminobenzimidazole
53. 1-(4-fluorobenzyl)-n-(1-(4-methoxyphenethyl)piperidin-4-yl)-1h-benzo[d]imidazol-2-amine
54. 1-(4-fluorobenzyl)-n-{1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl}-1h-benzimidazol-2-amine
55. 1-(p-fluorobenzyl)-2-[[1-(p-methoxyphenethyl)-4-piperidyl]amino]benzimidazole
56. 1-[(4-fluorophenyl)methyl]-n-(1-{2-[4-(methyloxy)phenyl]ethyl}piperidin-4-yl)-1h-benzimidazol-2-amine
57. 1-[(4-fluorophenyl)methyl]-n-{1-[2-(4-methoxyphenyl)ethyl]piperidin-4-yl}-1h-1,3-benzodiazol-2-amine
58. Ccris 7595
59. Hsdb 6799
60. Sr-01000003168
61. Hismanal (tn)
62. Einecs 272-441-9
63. Brn 4830190
64. Astemizole (jan/usp/inn)
65. Mjd-30
66. Astemizole,(s)
67. Astemizole-[d3]
68. Prestwick_35
69. Unii-7hu6337315
70. Mfcd00153919
71. Nsc 329963
72. Astemizole [usan:usp:inn:ban:jan]
73. Opera_id_62
74. Spectrum_000448
75. Astemizole [mi]
76. Astemizole [inn]
77. Astemizole [jan]
78. Prestwick0_000136
79. Prestwick1_000136
80. Prestwick2_000136
81. Prestwick3_000136
82. Spectrum2_001732
83. Spectrum3_001072
84. Spectrum4_001223
85. Spectrum5_001239
86. Astemizole [hsdb]
87. Astemizole [usan]
88. Astemizole [vandf]
89. Upcmld-dp024
90. Astemizole [mart.]
91. Cid_2247
92. Schembl4385
93. Astemizole [who-dd]
94. Regid_for_cid_2247
95. Bspbio_000212
96. Bspbio_002684
97. Kbiogr_001686
98. Kbioss_000928
99. 1h-benzimidazol-2-amine, 1-((4-fluorophenyl)methyl)-n-(1-(2-(4-methoxyphenyl)eth L)-4-piperidinyl)-
100. Astemizole, [o-methyl-3h]
101. Mls001148073
102. Divk1c_000039
103. Spectrum2300094
104. Spbio_001804
105. Spbio_002151
106. Bpbio1_000234
107. Gtpl2603
108. Astemizole, >=98% (hplc)
109. Dtxsid9020110
110. Upcmld-dp024:001
111. Bdbm24226
112. Hms500b21
113. Kbio1_000039
114. Kbio2_000928
115. Kbio2_003496
116. Kbio2_006064
117. Kbio3_001904
118. Astemizole [usp Impurity]
119. Ninds_000039
120. Hms1568k14
121. Hms1922l12
122. Hms2090f14
123. Hms2093l18
124. Hms2095k14
125. Hms2234m17
126. Hms3373b08
127. Hms3413n12
128. Hms3677n12
129. Hms3712k14
130. Pharmakon1600-02300094
131. Zinc601274
132. Bcp16088
133. Ex-a3350
134. Tox21_110680
135. Tox21_200095
136. Ccg-39453
137. Nsc329963
138. Nsc759570
139. Stl301859
140. Akos005064371
141. Tox21_110680_1
142. Db00637
143. Ks-5171
144. Nsc 759570
145. 1-[(4-fluorophenyl)methyl]-n-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidyl]benzimidazol-2-amine
146. Idi1_000039
147. Qtl1_000010
148. Ncgc00016913-01
149. Ncgc00016913-02
150. Ncgc00016913-03
151. Ncgc00016913-04
152. Ncgc00016913-05
153. Ncgc00016913-06
154. Ncgc00016913-07
155. Ncgc00016913-09
156. Ncgc00016913-10
157. Ncgc00016913-11
158. Ncgc00016913-12
159. Ncgc00016913-14
160. Ncgc00018288-01
161. Ncgc00022520-03
162. Ncgc00022520-04
163. Ncgc00022520-05
164. Ncgc00022520-06
165. Ncgc00022520-07
166. Ncgc00257649-01
167. Ac-36371
168. Hy-12532
169. Sbi-0051891.p002
170. Ab00052413
171. B7409
172. Cs-0011989
173. Ft-0622497
174. Ft-0662309
175. A13899
176. C06832
177. D00234
178. Ab00052413-18
179. Ab00052413_19
180. 844a779
181. L001015
182. Q423437
183. Sr-01000003168-2
184. Sr-01000003168-4
185. W-104654
186. Brd-k37249724-001-05-9
187. Brd-k37249724-001-16-6
188. Astemizole, United States Pharmacopeia (usp) Reference Standard
189. 1-(4-fluorobenzyl)-n-(1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl)-1h-benzimidazol-2-amine #
190. 1-(4-fluorophenylmethyl)-n-{1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl}-1h-benzimidazol-2-amine
191. Hismanal;1-[(4-fluorophenyl)methyl]-n-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1h-benzimidazol-2-amine
192. Xb7
| Molecular Weight | 458.6 g/mol |
|---|---|
| Molecular Formula | C28H31FN4O |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 458.24818979 g/mol |
| Monoisotopic Mass | 458.24818979 g/mol |
| Topological Polar Surface Area | 42.3 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 599 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Allergic Agents; Histamine H1 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antihistaminic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 147
Products containing astemizole were withdrawn from the US and Canadian markets by the manufacturer in June 1999.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 330
Antihistamines are indicated in the prophylactic and symptomatic treatment of perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis due to inhalant allergens and foods. /Antihistamines/ NOTE: Products containing astemizole were withdrawn from the US and Canadian markets by the manufacturer in June 1999.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 324
For more Therapeutic Uses (Complete) data for ASTEMIZOLE (7 total), please visit the HSDB record page.
Astemizole /has/ been shown to have a number of adverse effects on the electrophysiology of the heart, including altered repolarization, notched inverted T waves, prominent TU waves, prolonged QT interval, first- and second-degree AV block, ventricular tachycardia or fibrillation, and torsades de pointes.
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 623
Astemizole /has/ been recognized in rare cases to induce the syndrome of torsades de pointes, i.e. QT interval prolongation and life-threatening ventricular tachycardia. /It/ was found to prolong cardiac repolarization when its metabolic elimination was impaired, such as by liver disease or drugs that inhibit the 3A family of cytochrome P450. In vitro studies indicate that this action is due to blockade of one or more of the cardiac potassium channels that determine the duration of the action potential.
PMID:8725389 Woosley RL; Annu Rev Pharmacol Toxicol 36: 233-52 (1996)
Safe use of antihistamines during pregnancy has not been established; therefore, the drugs should not be used in women who are or may become pregnant unless the potential benefits justify the possible risks to the fetus. Some manufacturers caution that antihistamines should not be used during the third trimester because of the risk of severe reactions (e.g., seizures) to the drugs in neonates and premature infants. /Antihistamines/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 6
Patients receiving astemizole ... should be instructed to take the drug only as needed an not to exceed the prescribed dosage. The manufacturer of astemizole states that patients should be advised not to use astemizole on an as-needed ("prn") basis for immediate relief of symptoms. In addition, while a loading-dose regimen previously was recommended when an accelerated onset of effect was sought, such a regimen no longer is recommended because of the risk of cardiotoxicity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 6
For more Drug Warnings (Complete) data for ASTEMIZOLE (8 total), please visit the HSDB record page.
Astemizole was indicated for use in the relieving allergy symptoms, particularly rhinitis and conjunctivitis. It has been withdrawn from the market however due to concerns of arrhythmias.
Astemizole is a second generation H1-receptor antagonist. It does not significantly cross the blood brain barrier and therefore does not cause drowsiness or CNS depression at normal doses.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX11 - Astemizole
Absorption
Rapidly absorbed from the gastrointestinal tract.
Protein binding: 96%
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 325
Time to peak concentration: Within 1 hour.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 326
It is not known whether astemizole is distributed into human breast milk. Astemizole is distributed into the milk of dogs.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 327
Following concomitant administration of astemizole with food, oral bioavailability of the drug is decreased by 60%.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 11
Almost completely metabolized in the liver and primarily excreted in the feces.
Orally administered astemizole is well absorbed but undergoes an extensive first-pass metabolism to O-desmethylastemizole. Desmethylastemizole is formed in the human microsomal systems of the small intestine as well as the liver, which suggests the role of cytochromes P450 (P450s) in the first-pass metabolism of astemizole. Human P450s involved in the O-demethylation of astemizole have, however, not been identified, and the involvement of twelve known drug-metabolizing P450s were denied. During the course of the P450 identification study, higher activities of the astemizole O-demethylation in the rabbit small intestine than in the liver (about 3-fold) were found. These data suggest the possible involvement of CYP2J, since P450 included in this subfamily is dominantly expressed in the small intestine of rabbits. ...
PMID:12386130 Matsumoto S et al; Drug Metab Dispos 30 (11): 1240-5 (2002)
... Three metabolites of astemizole were detected in a /human/ liver microsomal system, i.e. desmethylastemizole (DES-AST), 6-hydroxyastemizole (6OH-AST) and norastemizole (NOR-AST) at the ratio of 7.4:2.8:1. Experiments with recombinant P450s and antibodies indicate a negligible role for CYP3A4 on the main metabolic route of astemizole, i.e. formation of DES-AST, although CYP3A4 may mediate the relatively minor metabolic routes to 6OH-AST and NOR-AST. Recombinant CYP2D6 catalyzed the formation of 6OH-AST and DES-AST. Studies with human liver microsomes, however, suggest a major role for a mono P450 in DES-AST formation. ...
PMID:11259984 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2014443 Matsumoto S, Yamazoe Y; Br J Clin Pharmacol 51 (2): 133-42 (2001)
Second-generation, relatively nonsedating histamine H1-receptor antagonists (H1-RA) are extensively used worldwide for the symptomatic treatment of allergic rhinoconjunctivitis and chronic urticaria. Information about the pharmacokinetics and pharmacodynamics of these medications, while still incomplete, is now sufficient to permit optimisation of therapy. Published pharmacokinetic and pharmacodynamic information on these H1-RA is summarised here, and areas where more data are required are delineated. Serum concentrations of most second-generation H1-RA are relatively low, and are usually measured by radioimmunoassay. After oral administration, peak concentrations are observed within 2 or 3 h. Bioavailability has not been well studied, due to the lack of intravenous formulations. Most H1-RA are metabolised in the hepatic cytochrome P450 system: terfenadine, astemizole, loratadine, azelastine, and ebastine have 1 or more active metabolites which are present in serum in higher concentrations than the respective parent compound, and therefore can be measured by high performance liquid chromatography. Cetirizine, an active metabolite of the first generation H1-receptor antagonist hydroxyzine, is not further metabolised to any great extent in vivo, and is eliminated via renal excretion. Levocabastine is also eliminated primarily by excretion. Serum elimination half-life values differ greatly from 1 H1-RA to another, and are 24 h or less for terfenadine, astemizole, loratadine, cetirizine, azelastine and ebastine, and the active metabolites of terfenadine, loratadine and ebastine. The active metabolite of azelastine (demethylazelastine) has a serum elimination half-life value of about 2 days, while that of astemizole (demethyl-astemizole) has a value of 9.5 days. From the few published studies in which the apparent volumes of distribution of the second-generation H1-RA have been calculated, it appears that tissue distribution is extensive. In children, the half-lives of H1-RA are generally shorter than are found in adults; there is no published information on the pharmacokinetics of astemizole, loratadine, azelastine, or ebastine in children. In some elderly adults, terfenadine, loratadine and cetirizine may have longer half-lives than in young healthy adults. There is little published data on the pharmacokinetics of the second-generation H1-RA in patients with impaired hepatic function. The half-life of cetirizine is prolonged in those with impaired renal function. There is a paucity of information on the pharmacokinetics of H1-RA in neonates, in pregnancy or during lactation.
PMID:1685361 Simons FE, Simons KJ; Clin Pharmacokinet 21 (5): 372-93 (1991)
The antiallergic effects of astemizole and its metabolites were studied in rats and guinea pigs. All of the metabolites of astemizole tested were more active than the parent compound in inhibiting contraction of the ileum and bronchoconstriction induced by histamine in guinea pigs. Desmethylastemizole was about the same as astemizole in inhibiting mepyramine binding in guinea pig cerebellum. In heterologous passive cutaneous anaphylaxis (PCA) and homologous PCA, the metabolites caused almost equipotent inhibition to that seen with astemizole. No H2-antagonistic activity was seen with astemizole or desmethylastemizole.
Kamei C et al; Arzneim Forsch 41 (9): 932-6 (1991)
Astemizole has known human metabolites that include 2-(4-hydroxyphenyl)acetaldehyde, 6-Hydroxyastemizole, 6-desmethylastermizole, and norastemizole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
1 day
Elimination: Astemizole (plus hydroxylated metabolites) - Mutiple doses, biphasic with an initial half life of 7 to 9 days (with plasma concentrations being reduced by 75% within this phase) and a terminal half life of about 19 days.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 325
With multiple doses: 7 to 9 days (initial); 19 days (terminal).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 330
The active metabolite of astemizole (demethyl-astemizole) has a value of 9.5 days.
PMID:1685361 Simons FE, Simons KJ; Clin Pharmacokinet 21 (5): 372-93 (1991)
Astemizole competes with histamine for binding at H1-receptor sites in the GI tract, uterus, large blood vessels, and bronchial muscle. This reversible binding of astemizole to H1-receptors suppresses the formation of edema, flare, and pruritus resulting from histaminic activity. As the drug does not readily cross the blood-brain barrier and preferentially binds at H1 receptors in the peripehery rather than within the brain, CNS depression is minimal. Astemizole may also act on H3-receptors, producing adverse effects.
Antihistamines are effective therapy against histamine-mediated conditions, including seasonal and perennial allergic rhinitis and chronic urticaria. They may also have a therapeutic role to play in asthma. Until recently all antihistamines produced some degree of drowsiness, as well as having anticholinergic side effects. Several non-sedating antihistamines have now been developed. Evidence suggests that their freedom from central nervous system effects is due to their lack of penetration of the blood brain barrier. They also have no appreciable binding to cholinergic receptors. Two of these non-sedating antihistamines, terfenadine and astemizole, have novel binding characteristics with the histamine H1 receptor, exhibiting irreversible binding at higher concentrations. In humans astemizole has a remarkably long half-life of elimination, on the order of 12 to 18 days for metabolites. Clinical trials have demonstrated that these newer antihistamines are as effective as classical antihistamines and that they have no greater incidence of central nervous system or anticholinergic side effects than placebo.
PMID:3147222 Kaliner MA, Check WA; Allergy Proc 9 (6): 649-63 (1988)
QT interval prolongation and torsade de pointes are associated with astemizole intake and have been ascribed to block the repolarizing K+ currents, specifically the rapidly activating component of the delayed rectifier iKr. ... The perforated patch clamp recording technique was used to study the effects of desmethylastemizole (20 nmol/liter) on action potentials and iKr in isolated rabbit ventricular myocytes. Desmethylastemizole produced action potential prolongation and the induction of plateau early afterdepolarizations. Under voltage clamp conditions, desmethylastemizole suppressed iKr amplitude by approximately 65%. The drug E-4031 (100 nmol/liter), which selectively blocks iKr, had a similar effect on current amplitude. Desmethylastemizole, the major astemizole metabolite, blocks the repolarizing K+ current iKr with high affinity. The clinical observation of QT prolongation and torsade de pointes found with astemizole intake may principally be caused by the proarrhythmic effects of its metabolite desmethylastemizole.
PMID:8917271 Vorperian VR et al; J Am Coll Cardiol 28 (6): 1556-61 (1996)
Astemizole /has/ been recognized in rare cases to induce the syndrome of torsades de pointes, i.e. QT interval prolongation and life-threatening ventricular tachycardia. /It/ was found to prolong cardiac repolarization when its metabolic elimination was impaired, such as by liver disease or drugs that inhibit the 3A family of cytochrome P450. In vitro studies indicate that this action is due to blockade of one or more of the cardiac potassium channels that determine the duration of the action potential.
PMID:8725389 Woosley RL; Annu Rev Pharmacol Toxicol 36: 233-52 (1996)