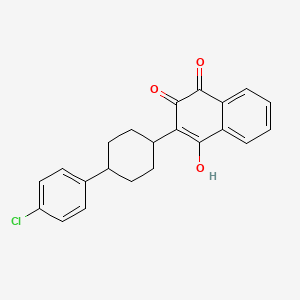

1. 2-(4-(4'-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone
2. 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone
3. 566c
4. 566c80
5. 566c80 Hydroxynaphthoquinone
6. Compound 566
7. Hydroxynaphthoquinone 566c80
8. Hydroxynaphthoquinone, 566c80
9. Mepron
10. Wellvone
1. 95233-18-4
2. Mepron
3. Wellvone
4. Acuvel
5. Atavaquone
6. 566c80
7. 94015-53-9
8. Cis-atovaquone
9. 137732-39-9
10. Mepron (antipneumocystic)
11. 566c
12. Bw 566c
13. 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxynaphthalene-1,2-dione
14. Atovaquone (atavaquone)
15. 2-(trans-4-(p-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone
16. F1w7quv0ki
17. 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone
18. Nsc-759582
19. 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone
20. 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione
21. Y883p1z2lt
22. Chebi:575568
23. 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxynaphthalene-1,4-dione
24. Ncgc00016961-01
25. Atovaquone 100 Microg/ml In Acetonitrile
26. 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione
27. Cas-95233-18-4
28. 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione
29. Dsstox_cid_2629
30. Trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione
31. 1,4-naphthalenedione, 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-, Trans-
32. 2-((1r,4r)-4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione
33. Dsstox_rid_76664
34. Dsstox_gsid_22629
35. 2-(cis-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthalenedione
36. 2-[trans-4-(p-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone
37. Cis-2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-1,4-naphthoquinone
38. 1,4-naphthalenedione, 2-(cis-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-
39. 1,4-naphthalenedione, 2-[cis-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-
40. 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione
41. Drg-0084
42. Bw 566c-80
43. Mepron (tn)
44. Bw-a 566c
45. Hsdb 7083
46. Sr-05000001438
47. Bw-566c-80
48. Atovacuona
49. Crl-8131 & Atovaquone
50. Unii-y883p1z2lt
51. Atovaquona
52. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone
53. Atovaquone & Interleukin 12
54. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-naphthalene-1,4-dione
55. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxynaphthalene-1,4-dione
56. Cis-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone
57. Trans-2-(4-(4-chlorophenyl) Cyclohexyl)-3-hydroxynaphthalene-1,4-dione
58. Ato & Il-12
59. Atovaquone [usan:usp:inn:ban]
60. Atovaquone-[d5]
61. Atovaquone- Bio-x
62. Bw 566c80
63. Spectrum_001743
64. Starbld0018905
65. Atovaquone [mi]
66. Specplus_000686
67. Atovaquone [inn]
68. Atovaquone [jan]
69. Prestwick0_000534
70. Prestwick1_000534
71. Prestwick2_000534
72. Prestwick3_000534
73. Spectrum2_001665
74. Spectrum3_000991
75. Spectrum4_001117
76. Spectrum5_001382
77. Unii-f1w7quv0ki
78. Atovaquone [hsdb]
79. Atovaquone [usan]
80. Atovaquone Ep Impurity B
81. Atovaquone [vandf]
82. Atovaquone [mart.]
83. Atovaquone [usp-rs]
84. Atovaquone [who-dd]
85. Schembl21694
86. Schembl21695
87. Atovaquone (jan/usp/inn)
88. Bspbio_000547
89. Bspbio_002681
90. Kbiogr_001594
91. Kbioss_002223
92. Atovaquone Related Compound A
93. Mls002153863
94. Bidd:gt0849
95. Divk1c_006782
96. Schembl637069
97. Spectrum1504210
98. Spbio_001849
99. Spbio_002468
100. Bpbio1_000603
101. Chembl222334
102. Chembl471792
103. Chembl519462
104. Gtpl9695
105. Schembl1542719
106. Schembl1649508
107. Schembl9975142
108. Schembl9975229
109. Atovaquone [orange Book]
110. Atovaquone, >=98% (hplc)
111. Cis-atovaquone (racemic)
112. Dtxsid7022629
113. Atovaquone For System Suitability
114. Chebi:95346
115. Kbio1_001726
116. Kbio2_002223
117. Kbio2_004791
118. Kbio2_007359
119. Kbio3_001901
120. Atovaquone [ep Monograph]
121. Dtxsid20916694
122. Atovaquone [usp Monograph]
123. Bdbm192009
124. Hms1569l09
125. Hms1922f19
126. Hms2089m14
127. Hms2093c10
128. Hms2096l09
129. Hms2235n08
130. Hms3369n09
131. Hms3651n20
132. Hms3713l09
133. Pharmakon1600-01504210
134. Amy15339
135. Bcp09477
136. Malarone Component Atovaquone
137. Tox21_110714
138. 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy-naphthalene-1,2-dione
139. Atovaquone Related Compound A [usp]
140. Ccg-39090
141. Fd7252
142. Mfcd00889188
143. Nsc759582
144. S3079
145. Stk636160
146. Trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone
147. Zinc12504271
148. 1,4-naphthalenedione, 2-(trans-4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-
149. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-dihydronaphthalene-1,4-dione
150. Akos005567953
151. Akos015895691
152. Akos015961933
153. Tox21_110714_1
154. Zinc100017856
155. Zinc100345537
156. Zinc116473771
157. Zinc299873031
158. Atovaquone Component Of Malarone
159. Bw-556c-80
160. Ccg-220534
161. Db01117
162. Nsc 759582
163. Ncgc00016961-02
164. Ncgc00016961-03
165. Ncgc00016961-04
166. Ncgc00016961-06
167. Ncgc00016961-07
168. Ncgc00016961-11
169. Ncgc00095113-01
170. Ncgc00095113-02
171. Ac-30251
172. As-12809
173. Ba164228
174. Hy-13832
175. Smr001233220
176. Sbi-0052893.p002
177. Ab00513855
178. Ft-0602868
179. Sw219222-1
180. Malarone Pediatric Component Atovaquone
181. A13708
182. Atovaquone Related Compound A [usp-rs]
183. C06835
184. D00236
185. F18448
186. Ab00053222-03
187. Ab00053222_04
188. Ab00053222_05
189. Atovaquone Component Of Malarone Pediatric
190. 233a184
191. A853147
192. Q418179
193. Atovaquone Related Compound A [usp Impurity]
194. Sr-05000001438-1
195. Sr-05000001438-2
196. Sr-05000001438-4
197. Sr-05000001438-5
198. Z1541632806
199. 2-[4-(4-chlorophenyl)cyclohexy]-3-hydroxy-1,4-naphthoquinone
200. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-napthoquinone
201. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1.4-naphthoquinone
202. 2-hydroxy-3-[4-(4-chlorophenyl)cyclohexyl]-1,4-naphthoquinone
203. 3-[4-(p-chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthoquinone
204. 1,2-naphthalenedione, 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy-
205. 1,4-naphthalenedione, 2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxy-
206. 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxynaphthoquinone, Trans-
207. 3-[4-(4-chlorophenyl)cyclohexyl]-4-hydroxy-1,2-naphthalenedione
208. Cis -2-(4-(4-chlorophenyl)cyclohexyl)-3-hydroxynaphthalene-1,4-dione
| Molecular Weight | 366.8 g/mol |
|---|---|
| Molecular Formula | C22H19ClO3 |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 366.1022722 g/mol |
| Monoisotopic Mass | 366.1022722 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 595 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Atovaquone |
| PubMed Health | Atovaquone (By mouth) |
| Drug Classes | Antiprotozoal |
| Drug Label | Atovaquone is an antiprotozoal agent. The chemical name of atovaquone is trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecular weight... |
| Active Ingredient | Atovaquone |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 750mg/5ml |
| Market Status | Prescription |
| Company | Amneal Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Mepron |
| PubMed Health | Atovaquone (By mouth) |
| Drug Classes | Antiprotozoal |
| Drug Label | MEPRON (atovaquone) is an antiprotozoal agent. The chemical name of atovaquone is trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecul... |
| Active Ingredient | Atovaquone |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 750mg/5ml |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 4 | |
|---|---|
| Drug Name | Atovaquone |
| PubMed Health | Atovaquone (By mouth) |
| Drug Classes | Antiprotozoal |
| Drug Label | Atovaquone is an antiprotozoal agent. The chemical name of atovaquone is trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecular weight... |
| Active Ingredient | Atovaquone |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 750mg/5ml |
| Market Status | Prescription |
| Company | Amneal Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Mepron |
| PubMed Health | Atovaquone (By mouth) |
| Drug Classes | Antiprotozoal |
| Drug Label | MEPRON (atovaquone) is an antiprotozoal agent. The chemical name of atovaquone is trans-2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecul... |
| Active Ingredient | Atovaquone |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 750mg/5ml |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Antipneumocystic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 149
A tablet containing a fixed dose of 250 mg of atovaquone and 100 mg of proguanil hydrochloride, taken orally, has been highly effective and safe in a 3-day regimen for treating mild to moderate attacks of chloroquine- and multidrug-resistant falciparum malaria.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1076
Atovaquone is indicated in the treatment of mild to moderate Pneumocystis carinii pneumonia (A-a gradient < or = 45 mmHg and pO2 > or = 60 mmHg on room air) in patients who are intolerant of sulfamethoxazole and trimethoprim combination. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 502
Atovaquone is indicated in the prevention of Pneumocystis carinii pneumonia in patients who are intolerant of sulfamethoxazole and trimethoprim combination. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 502
For more Therapeutic Uses (Complete) data for ATOVAQUONE (18 total), please visit the HSDB record page.
Patients should be advised regarding pulmonary manifestations of possibly concurrent bacterial, viral, fungal, or mycobacterial infections associated with HIV infection and/or progression of the underlying Pneumocystis carinii pneumonia and to contact a clinician if pulmonary symptomatology develops or worsens during atovaquone therapy. Clinical deterioration during atovaquone therapy could represent secondary infection with a nonsusceptible pathogen and/or progression of the underlying P. carinii pneumonia. All patients for whom atovaquone therapy is being considered should be evaluated carefully for other possible causes of pulmonary disease and treated with additional agents as appropriate.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 846
Fever was reported in up to 40% of patients receiving atovaquone in controlled clinical trials and occasionally has required discontinuance of the drug. Oral candidiasis was reported in 10%, cough in 25%, sweating in 10%, sinusitis in 7%, and rhinitis in 24% of patients receiving atovaquone in controlled clinical trials. Infection of dyspnea has occurred in 22 or 15% of patients receiving atovaquone. Hypotension, vortex keratopathy, transient sinus arrhythmia, increased serum creatine kinase (CK, creatine phosphokinase, CPK) concentrations, and transient conjunctivitis also have been reported rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 846
Hyperglycemia (exceeding 1.8 times the upper limit of normal) occurred in 9% of patients receiving atovaquone in controlled clinical trials. Hypoglycemia has occurred rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 846
Increased BUN and serum creatinine concentrations have been reported rarely in patients receiving atovaquone and occasionally have required discontinuance of the drug. Hyponatremia (less than 0.96 times the lower limit of normal range) was reported in up to 10% of patients receiving atovaquone in controlled clinical trials. Acute renal impairment has occurred in patients receiving atovaquone.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 846
For more Drug Warnings (Complete) data for ATOVAQUONE (13 total), please visit the HSDB record page.
For the treatment or prevention of Pneumocystis carinii pneumonia in patients who are intolerant to trimethoprim-sulfamethoxazole (TMP-SMX). Also indicated for the acute oral treatment of mild to moderate PCP in patients who are intolerant to TMP-SMX.
FDA Label
Atovaquone is a highly lipophilic drug that closely resembles the structure [ubiquinone]. Its inhibitory effect being comparable to ubiquinone, atovaquone can act by selectively affecting mitochondrial electron transport and parallel processes such as ATP and pyrimidine biosynthesis in atovaquone-responsive parasites. Cytochrome bc1 complex (complex III) seems to serve as a highly discriminating molecular target for atovaquone in Plasmodia. There is no significant risk for myelosuppression associated with atovaquone, making this drug a beneficial therapeutic agent for recipients of bone marrow transplantation.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
P01AX06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AX - Other agents against amoebiasis and other protozoal diseases
P01AX06 - Atovaquone
Absorption
The bioavailability of atovaquone is low and variable and is highly dependent on formulation and diet. Bioavailability of the suspension increases two-fold when administered with meals. When administered with food, bioavailability is approximately 47%. Without food, the bioavailability is 23%.
Route of Elimination
The half-life of atovaquone is long due to presumed enterohepatic cycling and eventual fecal elimination. There was little or no excretion of atovaquone in the urine (less than 0.6%).
Volume of Distribution
0.60 0.17 L/kg
Clearance
10.4 +/- 5.5 ml/min [HIV-infected patients receiving IV administration]
Drug absorption after a single oral dose is slow, erratic and variable; increased by 2- to 3-fold by fatty food; and dose-limited above 750 mg. More than 99% of the drug is bound to plasma protein, so its concentration in cerebrospinal fluid is less than 1% of that in plasma.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1076
The bioavailability of atovaquone is low and variable, and decreases significantly with single doses greater than 750 mg. A standard breakfast containing 23 g of fat has been shown to enhance absorption significantly. Oral suspension: The oral suspension provides a two-fold increase in bioavailability in fasting or fed conditions compared to the tablets. Bioavailability of the suspension increases two-fold when administered with meals; when administered with food, bioavailability is approximately 47%. Tablets: Bioavailability increases three-fold when administered with meals; bioavailability of the tablets when administered with food is approximately 23%.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 502
Fecal; > 94% of atovaquone was recovered in the feces over 21 days; < 0.6% was excreted in the urine.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 503
Bioavailability is increased with meals. Time to peak blood level is 1 to 8 hours, with a second peak in 24 to 96 hours.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 268
For more Absorption, Distribution and Excretion (Complete) data for ATOVAQUONE (8 total), please visit the HSDB record page.
Some evidence suggests limited metabolism (although no metabolites have been identified).
Atovaquone is not significantly metabolized by human beings. It is excreted in bile and more than 94% of the drug is recovered unchanged in feces; only traces appear in the urine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1076
Atovaquone is an antiprotozoal compound with good in vitro stability against metabolic inactivation. Previous human studies which did not involve radiolabelling had not accounted for a substantial proportion of the dose. The possible metabolism of atovaquone in men was examined in a radiolabelling study involving four healthy male volunteers. Radioactivity was eliminated almost exclusively via the feces. All radioactivity in plasma, urine, and feces was accounted for by atovaquone, with no evidence of metabolites. Radiolabelled atovaquone was administered to a patient with an indwelling biliary tube after surgery. Biliary radioactivity was approximately 10- to 40-fold higher than that in plasma and was accounted for by atovaquone. Atovaquone is not significantly metabolized in humans but is excreted into bile against a high concentration gradient.
PMID:9174191 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163907 Rolan PE et al; Antimicrob Agents Chemother 41(6): 1319-1321 (1997)
2.2 to 3.2 days
Atovaquone has a half-life averaging 1.5 to 3 days
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1076
2.2 to 3.2 days in adult patients with acquired immunodeficiency syndrome (AIDS), adult healthy volunteers, and immunocompromised children (ages 5 months to 13 years).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 502
The mechanism of action against Pneumocystis carinii has not been fully elucidated. In Plasmodium species, the site of action appears to be the cytochrome bc1 complex (Complex III). Several metabolic enzymes are linked to the mitochondrial electron transport chain via ubiquinone. Inhibition of electron transport by atovaquone will result in indirect inhibition of these enzymes. The ultimate metabolic effects of such blockade may include inhibition of nucleic acid and ATP synthesis. Atovaquone also has been shown to have good in vitro activity against Toxoplasma gondii.
Hydroxynaphthoquinone derivative that inhibits /parasitic/ mitochondrial electron transport.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 149
Atovaquone has possible cidal activity against susceptible organisms. The action against Pneumocystis carinii is not fully understood. Atovaquone is structurally similar to ubiquinone, which inhibits the mitochondrial electron-transport chain at the site of the cytochrome bc1 complex (complex III) in Plasmodium species. This may ultimately inhibit the synthesis of nucleic acid and ATP. Atovaquone also has been shown to have good in vitro activity against Toxoplasma gondii.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 502
At present, approaches to studying mitochondrial functions in malarial parasites are quite limited because of the technical difficulties in isolating functional mitochondria in sufficient quantity and purity. We have developed a flow cytometric assay as an alternate means to study mitochondrial functions in intact erythrocytes infected with Plasmodium yoelii, a rodent malaria parasite. By using a very low concentration (2 nM) of a lipophilic cationic fluorescent probe, 3,3'dihexyloxacarbocyanine iodide, we were able to measure mitochondrial membrane potential(DeltaPsim) in live intact parasitized erythrocytes through flow cytometry. The accumulation of the probe into parasite mitochondria was dependent on the presence of a membrane potential since inclusion of carbonyl cyanide m-chlorophenylhydrazone, a protonophore, dissipated the membrane potential and abolished the probe accumulation. We tested the effect of standard mitochondrial inhibitors such as myxothiazole, antimycin, cyanide and rotenone. All of them except rotenone collapsed the DeltaPsim and inhibited respiration. The assay was validated by comparing the EC50 of these compounds for inhibiting DeltaPsim and respiration. This assay was used to investigate the effect of various antimalarial drugs such as chloroquine, tetracycline and a broad spectrum antiparasitic drug atovaquone. We observed that only atovaquone collapsed DeltaPsim and inhibited parasite respiration within minutes after drug treatment. Furthermore, atovaquone had no effect on mammalian DeltaPsim. This suggests that atovaquone, shown to inhibit mitochondrial electron transport, also depolarizes malarial mitochondria with consequent cellular damage and death.
PMID:9020100 Srivastava IK et al; J Biol Chem 272(7): 3961-3966 (1997)