


1. Azadose
2. Azithromycin
3. Azithromycin Monohydrate
4. Azitrocin
5. Azythromycin
6. Cp 62993
7. Cp-62993
8. Cp62993
9. Dihydrate, Azithromycin
10. Goxal
11. Monohydrate, Azithromycin
12. Sumamed
13. Toraseptol
14. Ultreon
15. Vinzam
16. Zentavion
17. Zithromax
18. Zitromax
1. 117772-70-0
2. Vinzam
3. Toraseptol
4. Azithromycin (hydrate)
5. Azitro
6. 5fd1131i7s
7. 117772-70-0 (dihydrate)
8. Azithromycin Hydrate
9. Azitromax
10. Misultina
11. Tromix
12. Azadose
13. Ribotrex
14. Ultreon
15. Zenstavion
16. Azatek
17. Goxal
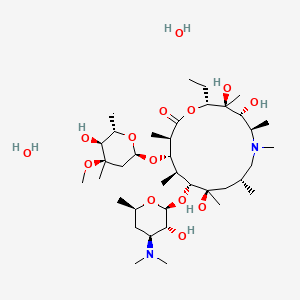
18. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-2-ethyl-3,4,10-trihydroxy-13-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one Dihydrate
19. Azithromycin (as Dihydrate)
20. Odaz
21. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-15-oxo-11-{[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy}-1-oxa-6-azacyclopentadecan-13-yl 2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranoside Dihydrate
22. Aciphar
23. Acitrocin
24. Azidromic
25. Azitral
26. Azitrix
27. Azitrom
28. Azitrox
29. Azitroxil
30. Azimix
31. Unii-5fd1131i7s
32. Azitrona Klonal
33. 9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin A Dihydrate
34. Azithromycin [mart.]
35. Schembl134723
36. Chebi:34546
37. Dtxsid80922539
38. Azithromycin Dihydrate 100 Microg/ml In Acetonitrile
39. Azithromycin Hydrate [jan]
40. Azithromycin Dihydrate [mi]
41. Ac-093
42. Hy-17506a
43. Azithromycin Dihydrate [vandf]
44. Akos015896370
45. Azithromycin Dihydrate [who-dd]
46. Azithromycin Dihydrate, >=98% (hplc)
47. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-13-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)-1-oxa-6-azacyclopentadecan-15-one Dihydrate
48. 1-oxa-6-azacyclopentadecan-15-one, 13-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)-, Dihydrate, (2r-(2r*,3s*,4r*,5r*,8r*,10r*,11r*,12s*,13s*,14r*))-
49. Azithromycin Dihydrate [orange Book]
50. Azithromycin Dihydrate [ep Monograph]
51. Azithromycin Dihydrate [usp Monograph]
52. 772a700
53. N-methyl-11-aza-10-deoxo-10-dihydro Erythromycin A
54. Q27116139
55. Azithromycin, United States Pharmacopeia (usp) Reference Standard
56. N-methyl-11-aza-10-deoxo-10-dihydroerythromycin A Dihydrate
57. Azithromycin, Pharmaceutical Secondary Standard; Certified Reference Material
58. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-2-ethyl-3,4,10-trihydroxy-13-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-onedihydrate
59. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-o
60. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one;dihydrate
61. 1-oxa-6-azacyclopentadecan-15-one, 13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-?-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-?-d-xylo-hexopyranosyl]oxy]-, Dihydrate, (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-
| Molecular Weight | 785.0 g/mol |
|---|---|
| Molecular Formula | C38H76N2O14 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 7 |
| Exact Mass | 784.52965510 g/mol |
| Monoisotopic Mass | 784.52965510 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)