

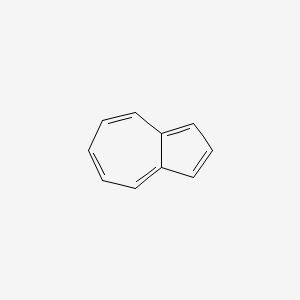

1. Azulekeep
2. Cyclopentacycloheptene
1. 275-51-4
2. Cyclopentacycloheptene
3. Azunamic
4. Bicyclo[5.3.0]decapentaene
5. Azulen
6. Bicyclo(5.3.0)decapentaene
7. Azulene, Homopolymer
8. Bicyclo(5.3.0)-1,3,5,7,9-decapentaene
9. Bicyclo(5.3.0)-deca-2,4,6,8,10-pentaene
10. 82r6m9mglp
11. Chebi:31249
12. Azulene (jan)
13. Nsc-89248
14. Azulene [jan]
15. Einecs 205-993-6
16. 82451-56-7
17. Mfcd00003810
18. Nsc 89248
19. Unii-82r6m9mglp
20. Azulene, 99%
21. Azulene [inci]
22. Azulene [mi]
23. Azulene [mart.]
24. Azulene [who-dd]
25. Bicyclo(5.3.0)-deca-1,3,5,7,9-pentaene
26. Azulene, Analytical Standard
27. Azusalen [as Sodium Sulfonate]
28. Chembl3272628
29. Dtxsid2059770
30. Hy-b0055
31. Nsc89248
32. Zinc1570209
33. Akos015840881
34. Cs-15638
35. Bicyclo[5.3.0]-1,5,7,9-decapentaene
36. Db-047243
37. A0634
38. Bicyclo(0.3.5)deca-1,3,5,7,9-pentaene
39. Cs-0006517
40. Ft-0622537
41. Azulene, Standard For Gc, >=99.0% (gc)
42. Bicyclo[5.3.0]deca-2,4,6,8,10-pentaene
43. C13408
44. D09768
45. A819116
46. Q144362
47. Sr-01000944574
48. J-016811
49. Sr-01000944574-1
50. Bicyclo-(0.3.5)-deca-1,3,5,7,9-pentaene
51. Bicyclo-(5.3.0)-deca-2,4,6,8,10-pentaene
| Molecular Weight | 128.17 g/mol |
|---|---|
| Molecular Formula | C10H8 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 128.062600255 g/mol |
| Monoisotopic Mass | 128.062600255 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 94.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)