

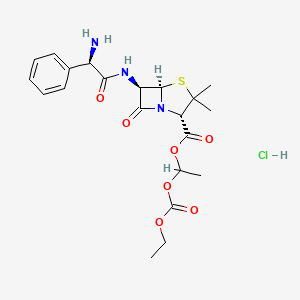

1. Bacampicillin
2. Bacampicillin Monohydrochloride
3. Bacampicine
4. Carampicillin
5. Ethoxycarbonyloxyethyl Ester Of Ampicillin
6. Penglobe
1. 37661-08-8
2. Bacampicillin Hcl
3. Spectrobid
4. Bacampicillin (hydrochloride)
5. Becampicillin Hydrochloride
6. Bapc
7. Chebi:2969
8. Pm034u953t
9. Nsc-758228
10. 37661-08-8 (hcl)
11. Dsstox_cid_25466
12. Dsstox_rid_80897
13. Dsstox_gsid_45466
14. (2s,5r,6r)-6-((r)-(2-amino-2-phenylacetamido))-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid Ester With Ethyl 1-hydroxyethyl Carbonate, Monohydrochloride
15. 1-ethoxycarbonyloxyethyl (2s,5r,6r)-6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate;hydrochloride
16. Ambacamp
17. Ambaxin
18. Bacacil
19. Velbacil
20. Bacampicillin Hydrochoride
21. Unii-pm034u953t
22. Spectrobid (tn)
23. Prestwick_776
24. Einecs 253-580-4
25. Bacampicillin Hydrochloride [usan:usp:jan]
26. Ncgc00016837-01
27. Surecn721373
28. Cas-37661-08-8
29. 4-propyloxybenzylamine
30. Mls002153801
31. Schembl124593
32. Chembl1200965
33. Dtxsid0045466
34. Hy-b1149a
35. Hms1569c14
36. Tox21_110640
37. Akos016014246
38. Tox21_110640_1
39. Ccg-220416
40. Cs-4754
41. Nsc 758228
42. Bacampicillin Hydrochloride [mi]
43. Bacampicillin Hydrochloride [jan]
44. Ncgc00179582-03
45. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, 1-((ethoxycarbonyl)oxy)ethyl Ester, Monohydrochloride, (2s-(2alpha,5alpha,6beta(s*)))-
46. Bacampicillin Hydrochloride (jp17/usan)
47. Bacampicillin Hydrochloride [usan]
48. Smr001233177
49. Bacampicillin Hydrochloride [mart.]
50. Bacampicillin Hydrochloride [vandf]
51. Bacampicillin Hydrochloride [who-dd]
52. C08123
53. D00927
54. Bacampicillin Hydrochloride [orange Book]
55. A823813
56. Bacampicillin Hydrochloride [ep Monograph]
57. Bacampicillin Hydrochloride [usp Impurity]
58. Q27105902
59. (2s,5r,6r)-6-[[(2r)-2-amino-1-oxo-2-phenylethyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid 1-ethoxycarbonyloxyethyl Ester Hydrochloride
60. 1-((ethoxycarbonyl)oxy)ethyl (2s-(2alpha,5alpha,6beta(s*)))-6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate Monohydrochloride
61. 1-ethoxycarbonyloxyethyl (2s,5r,6r)-6-[[(2r)-2-amino-2-phenyl-acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
62. 1-ethoxycarbonyloxyethyl (2s,5r,6r)-6-[[(2r)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate;hydron;chloride
63. 1-ethoxycarbonyloxyethyl (2s,5r,6r)-6-[[(2r)-2-azanyl-2-phenyl-ethanoyl]amino]-3,3-dimethyl-7-oxidanylidene-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Hydrochloride
64. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, 1-((ethoxycarbonyl)oxy)ethyl Ester, Hydrochloride
65. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-((aminophenylacetyl)amino)-3,3-dimethyl-7-oxo-, 1-((ethoxycarbonyl)oxy)ethyl Ester, Monohydrochloride, (2s-(2.alpha.,5.alpha.,6.beta.(s*)))-
| Molecular Weight | 502.0 g/mol |
|---|---|
| Molecular Formula | C21H28ClN3O7S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 501.1336491 g/mol |
| Monoisotopic Mass | 501.1336491 g/mol |
| Topological Polar Surface Area | 163 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 756 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)