
1. 14c-labeled Mupirocin
2. Bactroban
3. Brl 4910a
4. Brl-4910a
5. Brl4910a
6. Mupirocin Calcium
7. Mupirocin, 14c Labeled
8. Mupirocin, 14c-labeled
9. Mupirocin, Calcium Salt (2:1)
10. Mupirocin, Calcium Salt (2:1), Dihydrate
11. Mupirocin, Lithium Salt
12. Mupirocin, Sodium Salt
13. Pseudomonic Acid
14. Pseudomonic Acid A
1. 12650-69-0
2. Pseudomonic Acid
3. Bactroban
4. Mupirocine
5. Centany
6. Pseudomonic Acid A
7. Bactoderm
8. Mupirocina
9. Mupirocinum
10. Turixin
11. Brl 4910a
12. Brl-4910a
13. Trans-pseudomonic Acid
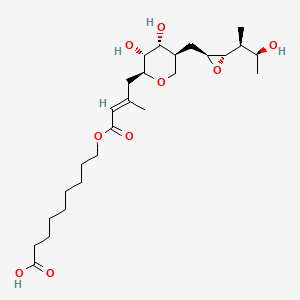
14. 9-[(e)-4-[(2s,3r,4r,5s)-3,4-dihydroxy-5-[[(2s,3s)-3-[(2s,3s)-3-hydroxybutan-2-yl]oxiran-2-yl]methyl]oxan-2-yl]-3-methylbut-2-enoyl]oxynonanoic Acid
15. Bactroban (tn)
16. Chembl719
17. Nsc-759182
18. Chebi:7025
19. D0gx863oa5
20. Mrc
21. Ncgc00164554-03
22. Plasimine
23. 80558-54-9
24. L-talo-non-2-enonic Acid, 5,9-anhydro-2,3,4,8-tetradeoxy-8-[[(2s,3s)-3-[(1s,2s)-2-hydroxy-1-methylpropyl]oxiranyl]methyl]-3-methyl-, 8-carboxyoctyl Ester, (2e)-
25. Mupirocine [french]
26. Mupirocinum [latin]
27. Mupirocina [spanish]
28. Bactroban Ointment
29. 9-(((e)-4-((2s,3r,4r,5s)-3,4-dihydroxy-5-(((2s,3s)-3-((2s,3s)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2h-pyran-2-yl)-3-methylbut-2-enoyl)oxy)nonanoic Acid
30. Centany (tn)
31. Mupirocin Neo-sensitabs
32. Mupirocin (usp/inn)
33. Sr-05000001947
34. Unii-d0gx863oa5
35. 1jzs
36. Mupirocin [usan:usp:inn:ban]
37. Mupirocin,(s)
38. 9-((e)-4-((2s,3r,4r,5s)-3,4-dihydroxy-5-(((2s,3s)-3-((2s,3s)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2h-pyran-2-yl)-3-methylbut-2-enoyloxy)nonanoic Acid
39. Mfcd01711620
40. Cpd000471888
41. Mupirocin [inn]
42. Mupirocin [mi]
43. Mupirocin [usan]
44. Mupirocin [vandf]
45. Mupirocin [mart.]
46. Mupirocin [usp-rs]
47. Mupirocin [who-dd]
48. Schembl3291
49. Dsstox_cid_26438
50. Dsstox_rid_81614
51. Dsstox_gsid_46438
52. 8-carboxyoctyl (e)-4-(2s,3r,4r,5s)-5-((2s,3s,4s,5s)-2,3-epoxy-5-hydroxy-4-methylhexyl)-3,4-dihydroxytetrahydro-2h-pyran-2-yl)-3-methylcrotonat
53. Mls001074711
54. Bidd:gt0320
55. Mupirocin, Pseudomonic Acid A
56. Mupirocin [green Book]
57. Cid_446596
58. Schembl1027618
59. Mupirocin [orange Book]
60. Dtxsid0046438
61. Mupirocin [ep Monograph]
62. Mupirocin [usp Impurity]
63. Chebi:94519
64. Gtpl10916
65. Mupirocin [usp Monograph]
66. Hms2234e20
67. Hms3259l05
68. Hms3712k03
69. Pharmakon1600-01505706
70. Hy-b0958
71. Zinc4102194
72. Tox21_112183
73. Bdbm50290686
74. Nsc759182
75. Nsc815348
76. Akos015994756
77. Mupirocin, >=92% (hplc), Powder
78. Ccg-213522
79. Db00410
80. Fd12069
81. Ks-5137
82. Nc00620
83. Nsc 759182
84. Nsc-815348
85. Ncgc00164554-05
86. Ncgc00164554-06
87. (e)-(2s,3r,4r,5s)-5-((2s,3s,4s,5s)-2,3-epoxy-5-hydroxy-4-methylhexyl)tetrahydro-3,4-dihydroxy-beta-methyl-2h-pyran-2-crotonic Acid, Ester With 9-hydroxynonanoic Acid
88. As-11580
89. Smr000471888
90. Sbi-0206892.p001
91. Cas-12650-69-0
92. M2955
93. S4297
94. D01076
95. M-8680
96. Ab01563109_01
97. Mupirocin, Antibiotic For Culture Media Use Only
98. Q413578
99. Sr-05000001947-1
100. Sr-05000001947-2
101. Brd-k15262564-001-06-9
102. Mupirocin, United States Pharmacopeia (usp) Reference Standard
103. (2e)-5,9-anhydro-2,3,4,8-tetradeoxy-8-(((2s,3s)-3-((1s,2s)-2-hydroxy-1-methylpropyl)oxiranyl)methyl)-3-methyl-l-talo-non-2-enonic Acid, 8-carboxyoctyl Ester
104. (e)-(2s,3r,4r,5s)-5-((2s,3s,4s,5s)-2,3-epoxy-5-hydroxy-4-methylhexyl)tetrahydro-3,4-dihydroxy-.beta.-methyl-2h-pyran-2-crotonic Acid, Ester With 9-hydroxynonanoic Acid
105. 9-((e)-4-((2s,3r,4r,5s)-3,4-dihydroxy-5-(((2s,3s)-3-((2s,3s)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)-tetrahydro-2h-pyran-2-yl)-3-methylbut-2-enoyloxy)nonanoic Acid
106. 9-((e)-4-{(2s,3r,4r,5s)-3,4-dihydroxy-5-[(2s,3s)-3-((1s,2s)-2-hydroxy-1-methyl-propyl)-oxiranylmethyl]-tetrahydro-pyran-2-yl}-3-methyl-but-2-enoyloxy)-nonanoic Acid
107. 9-({(2e)-4-[(2s,3r,4r,5s)-3,4-dihydroxy-5-({(2s,3s)-3-[(2s,3s)-3-hydroxybutan-2-yl]oxiran-2-yl}methyl)tetrahydro-2h-pyran-2-yl]-3-methylbut-2-enoyl}oxy)nonanoic Acid
108. 9-(4-((2s,3r,4r,5s)-3,4-dihydroxy-5-(((2s,3s)-3-((2s,3s)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)-tetrahydro-2h-pyran-2-yl)-3-methylbut-2-enoyloxy)nonanoic Acid
109. 9-(4-{3,4-dihydroxy-5-[3-(2-hydroxy-1-methyl-propyl)-oxiranylmethyl]-tetrahydro-pyran-2-yl}-3-methyl-but-2-enoyloxy)-nonanoic Acid (mupirocin)
110. 9-[(e)-4-[(2s,3r,4r,5s)-3,4-dihydroxy-5-[[(2s,3s)-3- [(2s,3s)-3-hydroxybutan-2-yl]oxiran-2-yl]methyl] Oxan-2-yl]-3-methylbut-2-enoyl]oxynonanoic Acid
111. 9-[(e)-4-[(2s,3r,4r,5s)-3,4-dihydroxy-5-[[(2s,3s)-3-[(1s,2s)-2-hydroxy-1-methyl-propyl]oxiran-2-yl]methyl]tetrahydropyran-2-yl]-3-methyl-but-2-enoyl]oxynonanoic Acid
112. 9-{[(2e)-4-[(2s,3r,4r,5s)-3,4-dihydroxy-5-{[(2s,3s)-3-[(2s,3s)-3-hydroxybutan-2-yl]oxiran-2-yl]methyl}oxan-2-yl]-3-methylbut-2-enoyl]oxy}nonanoic Acid
113. Nonanoic Acid, 9-((3-methyl-1-oxo-4-(tetrahydro-3,4-dihydroxy-5-((3-(2-hydroxy-1-methylpropyl)oxiranyl)methyl)-2h-pyran-2-yl)-2-butenyl)oxy)-, (2s-(2.alpha.(e),3.beta.,4.beta.,5.alpha.(2r*,3r*(1r*,2r*))))-
114. Nonanoic Acid, 9-((3-methyl-1-oxo-4-(tetrahydro-3,4-dihydroxy-5-((3-(2-hydroxy-1-methylpropyl)oxiranyl)methyl)-2h-pyran-2-yl)-2-butenyl)oxy)-, (2s-(2alpha(e),3beta,4beta,5alpha(2r*,3r*(1r*,2r*))))-
115. Rel-9-(((e)-4-((2s,3r,4r,5s)-3,4-dihydroxy-5-(((2s,3s)-3-((2s,3s)-3-hydroxybutan-2-yl)oxiran-2-yl)methyl)tetrahydro-2h-pyran-2-yl)-3-methylbut-2-enoyl)oxy)nonanoic Acid
| Molecular Weight | 500.6 g/mol |
|---|---|
| Molecular Formula | C26H44O9 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 17 |
| Exact Mass | 500.29853298 g/mol |
| Monoisotopic Mass | 500.29853298 g/mol |
| Topological Polar Surface Area | 146 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 694 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Bactroban |
| PubMed Health | Mupirocin |
| Drug Classes | Antibacterial, Antibiotic |
| Drug Label | BACTROBAN CREAM (mupirocin calcium cream, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (E,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy--methyl-... |
| Active Ingredient | Mupirocin calcium; Mupirocin |
| Dosage Form | Ointment; Cream |
| Route | Nasal; Topical |
| Strength | 2%; eq 2% base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 6 | |
|---|---|
| Drug Name | Centany |
| PubMed Health | Mupirocin (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Each gram of Centany (mupirocin ointment),2% contains 20 mg mupirocin in a soft white ointment base consisting of castor oil, oleyl alcohol, hard fat (Softisan 378) and propylene glycol monostearate. Mupirocin is a naturally occurring antibiotic. T... |
| Active Ingredient | Mupirocin |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Perrigo New York |
| 3 of 6 | |
|---|---|
| Drug Name | Mupirocin |
| PubMed Health | Mupirocin |
| Drug Classes | Antibacterial, Antibiotic |
| Drug Label | BACTROBAN CREAM (mupirocin calcium cream, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (E,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy--methyl-... |
| Active Ingredient | Mupirocin; Mupirocin calcium |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | eq 2% base; 2% |
| Market Status | Prescription |
| Company | Glenmark Pharms; Teva; Taro; Glenmark Generics; Fougera Pharms; Perrigo New York |
| 4 of 6 | |
|---|---|
| Drug Name | Bactroban |
| PubMed Health | Mupirocin |
| Drug Classes | Antibacterial, Antibiotic |
| Drug Label | BACTROBAN CREAM (mupirocin calcium cream, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (E,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy--methyl-... |
| Active Ingredient | Mupirocin calcium; Mupirocin |
| Dosage Form | Ointment; Cream |
| Route | Nasal; Topical |
| Strength | 2%; eq 2% base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 5 of 6 | |
|---|---|
| Drug Name | Centany |
| PubMed Health | Mupirocin (On the skin) |
| Drug Classes | Antibacterial |
| Drug Label | Each gram of Centany (mupirocin ointment),2% contains 20 mg mupirocin in a soft white ointment base consisting of castor oil, oleyl alcohol, hard fat (Softisan 378) and propylene glycol monostearate. Mupirocin is a naturally occurring antibiotic. T... |
| Active Ingredient | Mupirocin |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 2% |
| Market Status | Prescription |
| Company | Perrigo New York |
| 6 of 6 | |
|---|---|
| Drug Name | Mupirocin |
| PubMed Health | Mupirocin |
| Drug Classes | Antibacterial, Antibiotic |
| Drug Label | BACTROBAN CREAM (mupirocin calcium cream, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (E,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy--methyl-... |
| Active Ingredient | Mupirocin; Mupirocin calcium |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | eq 2% base; 2% |
| Market Status | Prescription |
| Company | Glenmark Pharms; Teva; Taro; Glenmark Generics; Fougera Pharms; Perrigo New York |
Indicated for the treatment of impetigo and secondary skin infections, leading to traumatic skin lesions, due to _Staphylococcus aureus_ and _Streptococcus pyogenes_.
Mupirocin is reported to be active against susceptible aerobic gram-positive cocci, such as _Staphylococcus aureus_, _Staphylococcus epidermidis_, and other beta-hemolytic streptococci_Streptococcus pyogenes_. It mediates its antibacterial activity by inhibiting the bacterial protein synthesis and formation of bacterial proteins essential for survival. The minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC). In one clinical study investigating the therapeutic effectiveness of topical mupirocin in impetigo, the therapeutic response rate was about 94 to 98% after one week following the end of therapy. In clinical studies of patients with primary and secondary skin infections, both elimination of the bacterial pathogen and clinical cure or improvement hav been demonstrated in over 90% of patients receiving topical mupirocin. Mupirocin resistance as high as 81% has been reported previously. Resistance to mupirocin, which occurs more frequently in methicillin-resistant than methicillin-susceptible staphylococci, may occur with the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX09 - Mupirocin
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AX - Other nasal preparations
R01AX06 - Mupirocin
Absorption
Systemic or percutaneous absorption of mupirocin following dermal application is expected to be minimal in adults and children. Occlusive dressings do not significantly enhance drug absorption, but damaged skin may allow enhanced penetration of the drug across the skin barrier.
Route of Elimination
Any mupirocin reaching the systemic circulation is rapidly metabolized to form the inactive monic acid, which is eliminated by renal excretion. Following the application of Centany (mupirocin ointment),2% to a 400 cm2 area on the back of 23 healthy volunteers once daily for 7 days, the mean (range) cumulative urinary excretion of monic acid over 24 hrs following the last administration was 1.25% (0.2% to 3.0%) of the administered dose of mupirocin.
Volume of Distribution
No information available.
Clearance
No information available.
Following intravenous or oral administration, mupirocin undergoes rapid hepatic metabolism to form the principal metabolite monic acid, which has no antibacterial activity.
In healthy male volunteers, the elimination half-life of mupirocin was about 20 to 40 minutes following intravenous administration. The elimination half-life of monic acid was about 30 to 80 minutes.
Mupirocin specifically and reversibly binds to bacterial isoleucyl transfer-RNA (tRNA) synthetase, which is an enzyme that promotes the conversion of isoleucine and tRNA to isoleucyl-tRNA. Inhibition of this enzyme subsequently leads to the inhibition of the bacterial protein and RNA synthesis. Mupirocin is bacteriostatic at lower concentrations but it exerts bactericidal effects with prolonged exposure, killing 90-99% of susceptible bacteria over a 24 hour period.