

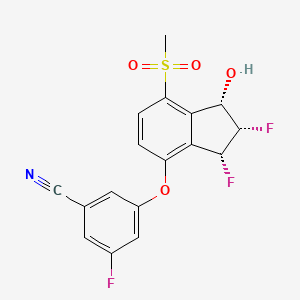

1. 3-(((1s,2s,3r)-2,3-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1h-inden-4-yl)oxy)-5-fluorobenzonitrile
2. Mk-6482
3. Mk6482
4. Pt-2977
5. Pt2977
6. Welireg
1. Pt2977
2. 1672668-24-4
3. Mk-6482
4. Welireg
5. Mk6482
6. Belzutifan [inn]
7. Belzutifan [usan]
8. Pt-2977
9. 3-(((1s,2s,3r)-2,3-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1h-inden-4-yl)oxy)-5-fluorobenzonitrile
10. 7k28nb895l
11. 3-[[(1s,2s,3r)-2,3-difluoro-1-hydroxy-7-methylsulfonyl-2,3-dihydro-1h-inden-4-yl]oxy]-5-fluorobenzonitrile
12. 3-{[(1s,2s,3r)-2,3-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1h-inden-4-yl]oxy}-5-fluorobenzonitrile
13. 3-((1s,2s,3r)-2,3-difluoro-1-hydroxy-7-methylsulfonylindan-4-yl)oxy-5-fluoro-benzonitrile
14. Benzonitrile, 3-(((1s,2s,3r)-2,3-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1h-inden-4-yl)oxy)-5-fluoro-
15. Belzutifan [who-dd]
16. Unii-7k28nb895l
17. Belzutifan [orange Book]
18. Chembl4585668
19. Schembl16560918
20. Gtpl11251
21. Pt 2977 [who-dd]
22. Bdbm373040
23. Dtxsid201334517
24. Pt 2977
25. Ex-a4441
26. Us9896418, Compound 289
27. Xrc66824
28. Nsc825217
29. Who 11196
30. At14994
31. Belzutifan (pt2977, Mk-6482)
32. Compound 2 [pmid: 31282155]
33. Nsc-825217
34. Mk-6482; Pt2977
35. Ac-35183
36. Hy-125840
37. Cs-0101119
38. A935088
39. Q27456641
40. 3-(((1s,2s,3r)-2,3-difluoro-1-hydroxy-7-(methanesulfonyl)-2,3-dihydro-1h-inden-4-yl)oxy)-5-fluorobenzonitrile
41. 3-((1s,2s,3r)-2,3-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1h-inden-4-yloxy)-5-fluorobenzonitrile
42. 72q
| Molecular Weight | 383.3 g/mol |
|---|---|
| Molecular Formula | C17H12F3NO4S |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 383.04391352 g/mol |
| Monoisotopic Mass | 383.04391352 g/mol |
| Topological Polar Surface Area | 95.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 675 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Belzutifan is indicated for the treatment of adult patients with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET), who do not require immediate surgery.
Belzutifan exerts its therapeutic effects by inhibiting a transcription factor necessary for the growth of solid tumors associated with VHL disease. It is taken once daily at approximately the same time each day, with or without food. Both severe anemia and hypoxia have been observed following therapy with belzutifan, and patients should be monitored closely before and during therapy to ensure patients can be managed as clinically indicated. There are no data regarding the use of erythropoiesis-stimulating agents for the treatment of belzutifan-induced anemia, and as such these therapies should be avoided. Belzutifan may cause embryo-fetal toxicity when administered to pregnant women. Female patients and male patients with female partners of reproductive potential should ensure that an effective form of contraception is used throughout therapy and for one week after the last dose - as belzutifan appears to decrease the efficacy of systemic hormonal contraceptives, patients should be advised to use an additional method of contraception (e.g. condoms) to eliminate the possibility of pregnancy during therapy.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX74 - Belzutifan
Absorption
In patients with VHL disease-associated renal cell carcinoma, the mean Cmax and AUC0-24h at steady-state - which was achieved after approximately three days of therapy - were 1.3 g/mL and 16.7 ghr/mL, respectively. The median Tmax is one to two hours following oral administration. The administration of belzutifan with food has a negligible effect on drug disposition - when given alongside a high-calorie, high-fat meal, the Tmax was delayed by approximately 2 hours with no other clinically meaningful effects observed.
Volume of Distribution
The steady-state volume of distribution of belzutifan following oral administration is approximately 130 L.
Clearance
The mean clearance of belzutifan following oral administration is 7.3 L/h.
Belzutifan is primarily metabolized by UGT2B17 and CYP2C19, and to a lesser extent by CYP3A4.
The mean elimination half-life of belzutifan is 14 hours.
Hypoxia-inducible factor 2 (HIF-2) is a transcription factor which aids in oxygen sensing by regulating genes that promote adaptation to hypoxia. In healthy patients, when oxygen levels are normal, HIF-2 is broken down via ubiquitin-proteasomal degradation by von-Hippel Lindau (VHL) proteins. In the presence of hypoxia, HIF-2 translocates into cell nuclei and forms a transcriptional complex with hypoxia-inducible factor 1 (HIF-1) - this complex then induces the expression of downstream genes associated with cellular proliferation and angiogenesis. Patients with von-Hippel Lindau (VHL) disease lack functional VHL proteins, leading to an accumulation of HIF-2, and this accumulation is what drives the growth of VHL-associated tumors. Belzutifan is an inhibitor of HIF-2 that prevents its complexation with HIF-1 in conditions of hypoxia or impaired VHL protein function, thereby reducing the expression of HIF-2 target genes and slowing/stopping the growth of VHL-associated tumors.