

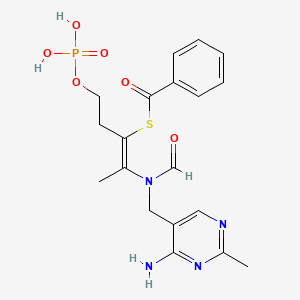

1. Benfothiamine
2. Benphothiamine
3. Btmp-benfo
4. Milgamma
5. Neurostop
6. S-benzoylthiamine Monophosphate
1. 22457-89-2
2. Benphothiamine
3. Betivina
4. Benzoylthiamine Monophosphate
5. S-benzoylthiamine O-monophosphate
6. S-benzoylthiamine Monophosphate
7. Benfotiamina
8. Benfotiaminum
9. 22457-89-2 (free Acid)
10. S-[(z)-2-[(4-amino-2-methylpyrimidin-5-yl)methyl-formylamino]-5-phosphonooxypent-2-en-3-yl] Benzenecarbothioate
11. (z)-s-(2-(n-((4-amino-2-methylpyrimidin-5-yl)methyl)formamido)-5-(phosphonooxy)pent-2-en-3-yl) Benzothioate
12. S-[2-{[(4-amino-2-methylpyrimidin-5-yl)methyl](formyl)amino}-5-(phosphonooxy)pent-2-en-3-yl] Benzenecarbothioate
13. Sr-01000872627
14. N-((4-amino-2-methyl-5-pyrimidinyl)methyl)-n-(4-hydroxy-2-mercapto-1-methyl-1-butenyl)formamide S-benzoate O-phosphate
15. Chebi:41039
16. Prestwick_68
17. Biotamin (tn)
18. Mfcd00057343
19. Prestwick2_000654
20. Prestwick3_000654
21. Benfotiamine (jan/inn)
22. Bspbio_000687
23. Schembl188070
24. Bpbio1_000757
25. Chembl4303665
26. Schembl19184708
27. Hms1570c09
28. Hms2097c09
29. Hms3714c09
30. Zinc2015559
31. Stl453586
32. Akos015920320
33. Ac-8280
34. Ccg-220654
35. Db11748
36. Ncgc00179477-01
37. (3z)-4-{n-[(4-amino-2-methylpyrimidin-5-yl)methyl]carbonylamino}-3-(phenylcarb Onylthio)pent-3-enyl Dihydrogen Phosphate
38. 57b892
39. A15020
40. D01255
41. Sr-01000872627-1
42. Sr-01000872627-2
43. 137-74-6
44. S-[(2z)-2-{[(4-amino-2-methylpyrimidin-5-yl)methyl](formyl)amino}-5-(phosphonooxy)pent-2-en-3-yl] Benzenecarbothioate
45. S-{(1z)-2-[[(4-amino-2-methyl-5-pyrimidinyl)methyl](formyl)amino]-1-[2-(phosphonooxy)ethyl]-1-propenyl} Benzenecarbothioate
46. S-{(1z)-2-[[(4-amino-2-methyl-5-pyrimidinyl)methyl](formyl)amino]-1-[2-(phosphonooxy)ethyl]-1-propenyl} Benzenecarbothioate, Aldrichcpr
| Molecular Weight | 466.4 g/mol |
|---|---|
| Molecular Formula | C19H23N4O6PS |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 10 |
| Exact Mass | 466.10759264 g/mol |
| Monoisotopic Mass | 466.10759264 g/mol |
| Topological Polar Surface Area | 181 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 697 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11D - Vitamin b1, plain and in combination with vitamin b6 and b12
A11DA - Vitamin b1, plain
A11DA03 - Benfotiamine