

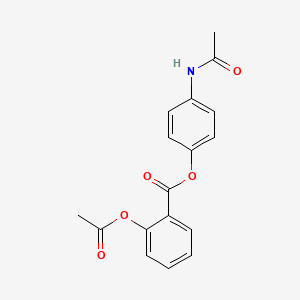

1. 2-acetoxy-4'-acetaminophenylbenzoate
2. 4-acetamidophenyl-o-acetylsalicylate
3. Benoral
4. Benorylate
5. Salipran
1. 5003-48-5
2. Benorylate
3. 4-acetamidophenyl 2-acetoxybenzoate
4. Salipran
5. Benoral
6. Benortan
7. Fenasprate
8. Fenasparate
9. Aspirin Acetaminophen Ester
10. Benorilato
11. Win 11450
12. Benzoic Acid, 2-(acetyloxy)-, 4-(acetylamino)phenyl Ester
13. 4-acetamidophenyl Salicylate Acetate
14. P-acetamidophenyl Acetylsalicylate
15. P-n-acetylaminophenylacetylsalicylate
16. To 125
17. (4-acetamidophenyl) 2-acetyloxybenzoate
18. 2-acetoxy-4'-(acetamino)phenylbenzoate
19. 4'-(acetamido)phenyl-2-acetoxybenzoate
20. W1qx9dv96g
21. 2-(acetyloxy)benzoic Acid 4-(acetylamino)phenyl Ester
22. Benorilate (inn)
23. Win-11450
24. Salicylic Acid, Acetate, Ester With 4'-hydroxyacetanilide
25. Ncgc00167468-01
26. Benorilate 100 Microg/ml In Acetonitrile
27. Benorilate [inn]
28. 4-(acetylamino)phenyl 2-(acetyloxy)benzoate
29. Dsstox_cid_2649
30. Dsstox_rid_76674
31. Dsstox_gsid_22649
32. Benorilato [spanish]
33. Benorilatum
34. Quinexin
35. Benorilatum [inn-latin]
36. Benorilato [inn-spanish]
37. Cas-5003-48-5
38. Ccris 1739
39. Einecs 225-674-5
40. Unii-w1qx9dv96g
41. Brn 2166628
42. Benorilate [inn:ban:dcf]
43. Benorilate,(s)
44. Benorylate [mi]
45. Benorilate [mart.]
46. Benorilate [who-dd]
47. Schembl25167
48. 2-(acetyloxy)-benzoicacid 4-(acetylamino)phenylester
49. Chembl162036
50. Zinc1003
51. Dtxsid5022649
52. Chebi:135340
53. Bcpp000346
54. Bcp13353
55. Tox21_112472
56. Mfcd00864257
57. S5043
58. Akos016010035
59. Tox21_112472_1
60. Ccg-267615
61. Db13657
62. Ks-5206
63. Ncgc00167468-02
64. Ac-12036
65. As-13290
66. Bcp0726000190
67. Hy-107795
68. Cs-0030669
69. Ft-0740917
70. 4-(acetylamino)phenyl 2-(acetyloxy)benzoate #
71. A18816
72. D07291
73. Q373243
74. Sr-01000944978
75. J-514276
76. Sr-01000944978-1
| Molecular Weight | 313.30 g/mol |
|---|---|
| Molecular Formula | C17H15NO5 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 313.09502258 g/mol |
| Monoisotopic Mass | 313.09502258 g/mol |
| Topological Polar Surface Area | 81.7 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA10 - Benorilate