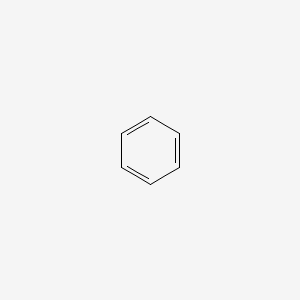

1. Benzol
2. Benzole
3. Cyclohexatriene
1. Benzol
2. 71-43-2
3. Cyclohexatriene
4. Benzole
5. Pyrobenzole
6. Benzine
7. Benzen
8. Phenyl Hydride
9. Coal Naphtha
10. Pyrobenzol
11. Phene
12. Mineral Naphtha
13. Bicarburet Of Hydrogen
14. Benzolene
15. Benzin
16. [6]annulene
17. Motor Benzol
18. Carbon Oil
19. Benzeen
20. Benzolo
21. Fenzen
22. Nitration Benzene
23. (6)annulene
24. Benzol 90
25. Nci-c55276
26. Rcra Waste Number U019
27. Benzinum
28. 1,3,5-cyclohexatriene
29. Nsc 67315
30. Un 1114
31. Chebi:16716
32. Chembl277500
33. Mfcd00003009
34. Nsc-67315
35. J64922108f
36. Benzeen [dutch]
37. Benzen [polish]
38. Fenzen [czech]
39. Benzolo [italian]
40. Bnz
41. Benzine (obs.)
42. Benzin (obs.)
43. Caswell No. 077
44. Benzol Diluent
45. Benzene 100 Microg/ml In Methanol
46. Benzene, Acs Reagent, >=99.0%
47. Benzene, Pure
48. Ccris 70
49. 54682-86-9
50. Hsdb 35
51. Einecs 200-753-7
52. Un1114
53. Epa Pesticide Chemical Code 008801
54. Benzolum
55. Aromatic Alkane
56. Benzene (including Benzene From Gasoline)
57. P-benzene
58. Benzene Solution
59. Benzene-
60. Ai3-00808
61. C6h6
62. 26181-88-4
63. 1hyz
64. 1swi
65. Unii-j64922108f
66. [6]-annulene
67. Benzene Acs Grade
68. Benzene, For Hplc
69. {[6]annulene}
70. Ph-h
71. Phenyl; Phenyl Radical
72. 2z9g
73. 4i7j
74. Benzene [vandf]
75. Benzinum [hpus]
76. Benzene + Aniline Combo
77. Benzene [hsdb]
78. Benzene [iarc]
79. Benzene (benzol)
80. Benzene [mi]
81. Benzene [mart.]
82. Dsstox_cid_135
83. Benzene, Labeled With Carbon-14 And Tritium
84. Wln: Rh
85. Benzene [usp-rs]
86. Benzene [who-dd]
87. Epitope Id:116867
88. Benzene, Purification Grade
89. Ec 200-753-7
90. Benzene, Analytical Standard
91. Dsstox_rid_79433
92. Benzene, Lr, >=99%
93. Dsstox_gsid_39242
94. Ghl.pd_mitscher_leg0.503
95. Benzene, Anhydrous, 99.8%
96. Benzene, Ar, >=99.5%
97. Dtxsid3039242
98. 3,4-dnh
99. 1l83
100. 220l
101. 223l
102. Benzene 10 Microg/ml In Methanol
103. Zinc967532
104. Trans-n-methylphenylcyclopropylamine
105. Act02832
106. Bcp26158
107. Benzene 20 Microg/ml In Triacetin
108. Benzene, For Hplc, >=99.8%
109. Benzene, For Hplc, >=99.9%
110. Nsc67315
111. Tox21_202487
112. 1,3-cyclohexadiene-5,6-diylradical
113. Bdbm50167939
114. Bm 613
115. Stl264205
116. Benzene 1000 Microg/ml In Methanol
117. Benzene 5000 Microg/ml In Methanol
118. Benzene, Purum, >=99.0% (gc)
119. Akos008967253
120. Benzene, Saj First Grade, >=99.0%
121. Cas-71-43-2
122. Acetone Impurity C [ep Impurity]
123. Benzene [un1114] [flammable Liquid]
124. Benzene, Jis Special Grade, >=99.5%
125. Erythro-phenyl-2-piperidyl-carbinol,(-)
126. Ncgc00090744-01
127. Ncgc00090744-02
128. Ncgc00163890-01
129. Ncgc00163890-02
130. Ncgc00260036-01
131. Trans-n, N-dimethylphenylcyclopropylamine
132. Cc-34,(+/-)
133. Rng
134. Ds-002542
135. B0020
136. Ft-0622636
137. Ft-0622637
138. Ft-0622667
139. Ft-0627856
140. Ft-0657604
141. Q0038
142. Q2270
143. Benzene 30 Microg/ml In N,n-dimethylacetamide
144. Benzene, Acs Spectrophotometric Grade, >=99%
145. C01407
146. Benzene, Reagentplus(r), Thiophene Free, >=99%
147. Benzene, Puriss. P.a., Reag. Ph. Eur., >=99.7%
148. Q26841227
149. Z57120059
150. Biperiden Hydrochloride Impurity F [ep Impurity]
151. Benzene, For Residue Analysis, Suitable For 5000 Per Jis
152. Benzene, Suitable For 300 Per Jis, >=99.5%, For Residue Analysis
153. Benzene, Pharmaceutical Secondary Standard; Certified Reference Material
154. Benzene, Suitable For 1000 Per Jis, >=99.5%, For Residue Analysis
155. Benzene, Puriss., Absolute, Over Molecular Sieve (h2o <=0.005%), >=99.5% (gc)
156. 25053-22-9
| Molecular Weight | 78.11 g/mol |
|---|---|
| Molecular Formula | C6H6 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 78.0469501914 g/mol |
| Monoisotopic Mass | 78.0469501914 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 15.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): Has been used as a disinfectant. /Former Use/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 188
Protected intercourse may be prudent following high exposure to benzene. As well, nursing mothers may be advised to discontinue nursing for 5 days following high exposure.
Zenz, C., O.B. Dickerson, E.P. Horvath. Occupational Medicine. 3rd ed. St. Louis, MO., 1994, p. 712
Immediately dangerous to life and health = 500 ppm
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 754
... It has been estimated that 5-10 minutes of exposure to 20,000 ppm benzene in air is usually fatal.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Benzene p.30 PB2008-100004 (2007). Available from, as of August 12, 2014: https://www.atsdr.cdc.gov/toxprofiles/index.asp
Estimated oral doses from 9-30 g have proved fatal.
WHO; Environmental Health Criteria 150: Benzene p.46 (1993)
Single exposures to concentrations of 66,000 mg/cu m (20,000 ppm) commercial benzene have been reported to be fatal in man within 5-10 minutes. At lower levels, loss of consciousness, irregular heart-beat, dizziness, headache and nausea are observed.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 116 (1982)
Benzene is readily absorbed via lung, & about 40-50% is retained. ... It is taken up preferentially by fatty & nervous tissues, & about 30-50% ... is excreted unchanged via lung; a 3-phase excretion pattern is seen at ... /approx/ 0.7-1.7 hr, 3-4 hr, & 20-30 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V7 211 (1974)
When benzene was placed on skin under closed cup it was absorbed at rate of 0.4 mg/sq cm/hr (Hanke et al 1961) ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 117 (1982)
Mice treated SC with 2 mL (3)H-labeled benzene/kg contained irreversibly bound radioactivity with decreasing binding magnitude in the following organs: liver, brain, kidney, spleen, fat. Mice treated with 2 daily SC doses of 0.5 mL (3)H-benzene/kg for 1-10 days showed a radioactivity binding with liver & bone marrow residues which increased with treatment duration, except in the case of binding to bone marrow which decreased after day 6.
PMID:663402 Snyder R et al; Res Commun Chem Pathol Pharmacol 20 (1): 191-4 (1978)
When administered to mice subcutaneously, 72% of dose is recovered in expired air.
PMID:849319 Andrews LS et al; Biochem Pharmacol 26: 293 (1977)
For more Absorption, Distribution and Excretion (Complete) data for BENZENE (14 total), please visit the HSDB record page.
The major metabolitesof benzene metabolism are phenol, hydroquinone, and catechol. These metabolites are interactive and can affect the rate of each other's metabolism because they are substrates for the P-450 enzyme system. The route of exposure has little effect on the subsequent metabolism of benzene to hemotoxic metabolites.
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 754
Metabolic products in rat ... are phenol, hydroquinone, catechol, hydroxyhydroquinone, & phenylmercapturic acid. Conjugated phenols have been reported ... except for a small amt of free phenol, all the phenolic metabolites were excreted in conjugated form. When (3)H-benzene was admin to mice, (3)H2O was also recovered from urine.
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 688
Yields N-acetyl-S-phenyl-cysteine in rat. Yields benzyl alcohol in guinea pigs. ... Yields cis-1,2-dihydro-1,2-dihydroxybenzene in pseudomonas. Phenol in pseudomonas & achromobacter. Yields cis,cis-muconic acid in rabbit. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. B-4
In the rabbit, the major hydroxylation product of benzene was phenol, which along with some catechol and hydroquinone, was found in the urine conjugated with ethereal sulfate or glucuronic acid.
USEPA; Ambient Water Quality Criteria: Benzene p.C-11 (1980) EPA 440/5-80-018
For more Metabolism/Metabolites (Complete) data for BENZENE (26 total), please visit the HSDB record page.
Benzene has known human metabolites that include phenol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Whole body: 9-24 hours; however, up to 90 hours due to distribution in fat; [TDR, p. 154]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 154
The excretion of unchanged benzene from the lung of rats was reported to be biphasic, suggesting a two-compartment model for distribution and a half-life of 0.7 hr. This agreed with experimental half-life values for various tissues that ranged from 0.4 to 1.6 hr.
Rickert DE et al; Toxicol Appl Pharmacol 49: 417 (1979) as cited in USEPA; Ambient Water Quality Criteria: Benzene p.C-11 (1980) EPA 440/5-80-018
... The half-time of benzene in /high lipid content/ tissues is approximately 24 hours.
Zenz, C., O.B. Dickerson, E.P. Horvath. Occupational Medicine. 3rd ed. St. Louis, MO., 1994, p. 146
Covalent interaction of a benzene metabolite with dna was shown in vivo, but no information was given about the chem nature of this metabolite. A likely intermediate in benzene metabolism is benzene oxide. In neutral aq media it rearranges only slowly to the phenol so that its lifetime could be long enough for diffusion from the site of activation to the dna. Alternatively, the metabolic appearance of polyhydroxy derivatives suggests the formation of a phenol epoxide, so that the reactive molecule could be a secondary metabolite.
PMID:890848 Lutz WK, Schlatter C; Chem Biol Interact 18 (2): 241-6 (1977)
The available evidence supports the concept that benzene toxicity is caused by one or more metabolites of benzene. ... Benzene metabolites containing 2 or 3 hydroxyl groups inhibited mitosis. Toluene, which inhibits benzene metabolism, protected animals against benzene-induced myelotoxicity. Benzene toxicity could be correlated with the appearance of benzene metabolites in bone marrow. Although it is clear that benzene can be metabolized in bone marrow, the observation that partial hepatectomy protects against benzene toxicity suggests that a metabolite formed in liver is essential for benzene toxicity.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 113 (1982)
... Importance of polyhydroxylated derivatives of benzene & their semiquinones. ... /It has been/ shown that hydroquinone inhibits rat brain microtubule polymerization; that hydroquinone & para-benzoquinone are the most potent inhibitors of T- & B-lymphocyte function, as measured in mouse spleen cells in culture; that hydroquinone inhibits lectin-stimulated lymphocyte agglutination in rat spleen prepn in vitro; & that para-benzoquinone is the metabolite most likely to be responsible for suppression of lymphocyte transformation & microtubule assembly in rat spleen cells in culture. However, admin of these cmpd to animals does not produce the typical picture of benzene toxicity ... admin /of/ major metabolites of benzene to mice ... failed to ... decr ... red blood cell production, using the (59)Fe uptake technique ... /it's been/ suggested that ring-opening products may play a role in benzene toxicity. ... In mice benzene treatment suppressed subsequent colony forming unit-C formation from bone-marrow cells in vitro. Treating the animals with phenol, hydroquinone or benzene dihydrodiol failed to suppress colony forming unit-C. Thus, the toxic metabolites of benzene have yet to be identified.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 113 (1982)
... Radioactivity /has been demonstrated/ in a nucleic acid fraction from rat liver following admin of either (3)h- or (14)C-labelled benzene. It has been shown that benzene binds covalently to protein in liver, bone marrow, kidney, lung, spleen, blood, & muscle. Less covalent binding was observed to the protein of bone marrow, blood, & spleen of C57Bl/6 mice, which are more resistant to the benzene-induced effects on red cell production, than to that of sensitive DBA/2 mice. ... Covalent binding of benzene to protein in perfused bone-marrow prepn /has been demonstrated/. ... A metabolite of phenol binds to liver protein more efficiently than does benzene oxide, & they have electrophoretically separated hepatic proteins to which benzene preferentially binds. ... Covalent binding to mitochondria is a prominent feature of benzene metabolism. ... There is relatively more radioactivity in a nucleic acid-rich fraction of a benzene metabolite isolated from mouse bone-marrow cells than in a similar fraction from liver.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V29 113 (1982)
For more Mechanism of Action (Complete) data for BENZENE (12 total), please visit the HSDB record page.