
1. Bencetonium Chloride
2. Benzethonium Chloride
3. Chloride, Bencetonium
4. Chloride, Benzethonium
5. Formula Magic
6. Hyamine 1622
7. Magic, Formula
8. Orchid Fresh Ii
9. Phemeride
10. Phemerol
11. Phemethryn
12. Puri Clens
13. Puri-clens
14. Puriclens
15. Quatrachlor
16. Solamin
1. Sanizol
2. Benzethonium Ion
3. 10172-60-8
4. Benzethonium Cation
5. 1vu15b70bp
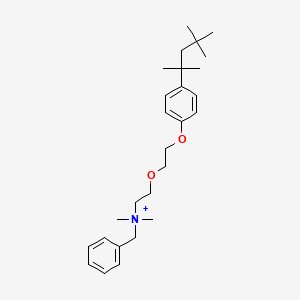
6. Benzyl-dimethyl-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethyl]azanium
7. Benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)ammonium
8. Ammonium, Benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-
9. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)
10. Chembl221753
11. Benzyl-dimethyl-[2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]ammonium
12. Nsc20200
13. Ncgc00016373-03
14. Cas-121-54-0
15. Unii-1vu15b70bp
16. Benzothonium
17. Bztcl
18. N-benzyl-n,n-dimethyl-2-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)ethoxy)ethanaminium
19. N-benzyl-n,n-dimethyl-2-{2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy}ethanaminium
20. Spectrum_000072
21. Benzethonium Hydrochloride
22. Prestwick0_000708
23. Prestwick1_000708
24. Prestwick2_000708
25. Prestwick3_000708
26. Spectrum2_000134
27. Spectrum3_000313
28. Spectrum4_000248
29. Spectrum5_000858
30. Cid_8478
31. Benzothonium [vandf]
32. Bspbio_000895
33. Bspbio_001906
34. Kbiogr_000656
35. Kbioss_000472
36. Benzethonium [who-dd]
37. Divk1c_000775
38. Schembl122985
39. Spbio_000208
40. Spbio_002816
41. Bpbio1_000985
42. Chembl1182210
43. Dtxsid5046984
44. Chebi:94725
45. Kbio1_000775
46. Kbio2_000472
47. Kbio2_003040
48. Kbio2_005608
49. Kbio3_001406
50. Ninds_000775
51. Zinc1571009
52. Bdbm50203812
53. Stl256857
54. Akos022098568
55. Db11125
56. Idi1_000775
57. Qtl1_000012
58. Ncgc00016373-01
59. Ncgc00016373-02
60. Ncgc00016373-04
61. Ncgc00016373-05
62. Ncgc00016373-07
63. Ncgc00016373-09
64. Nci60_001693
65. Sbi-0051292.p003
66. Ab00053793
67. A19421
68. Ab00053793_12
69. Ab00053793_13
70. Brd-k72723676-003-10-3
71. Q27166518
72. 2-(2-(4-diisobutylphenoxy)ethoxy)ethyl)dimethylbenzylammoniumchloride
73. Dimethyl-(phenylmethyl)-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethyl]ammonium
74. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-
| Molecular Weight | 412.6 g/mol |
|---|---|
| Molecular Formula | C27H42NO2+ |
| XLogP3 | 6.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 12 |
| Exact Mass | 412.321554582 g/mol |
| Monoisotopic Mass | 412.321554582 g/mol |
| Topological Polar Surface Area | 18.5 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 1 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated as an antiseptic agent. No therapeutic indications for clinical use.
Benzethonium belongs to the family of compounds known as cationic detergents that act by disrupting lipid bilayers. It demonstrated antitumor activity against cancer cell lines in vitro. The effective dose required to decrease cell viability by 50% after 48 hours (ED50) of benzethonium for FaDu (hypopharyngeal squamous cancer) and C666-1 (nasopharyngeal cancer) cell lines were approximately 3.8 and 5.3 mol/L, respectively.
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA09 - Benzethonium
Absorption
It is reported that percutaneous absorption of benzethonium is clinically insignificant. Benzethonium chloride belongs to the family of
Route of Elimination
Pharmacokinetic studies on benzethonium have not been conducted.
Volume of Distribution
Pharmacokinetic studies on benzethonium have not been conducted.
Clearance
Pharmacokinetic studies on benzethonium have not been conducted.
Pharmacokinetic studies on benzethonium have not been conducted.
Pharmacokinetic studies on benzethonium have not been conducted.
While exact mechanism of benzethonium is not fully understood, it is proposed that benzethonium acts similarly to other structurally-related quaternary ammonium compounds in disrupting cytoplasmic and outer membrane lipid bilayers of microorganisms. The positively charged quaternary nitrogen associates with the polar head groups of acidic membrane phospholipids, followed by the hydrophobic tail interacting with the hydrophobic membrane core. Benzethonium is thought to form mixed-micelle aggregates with hydrophobic membrane components that solubilize membrane and lyse the cells, leading to leakage of cytoplasmic contents. Based on findings in vitro cell assays, its mode of action on cancer cells may involve cancer cell apoptosis via dysregulating mitochondria or rough endoplasmic reticulum (rER). It is proposed that intracellular cationic molecules such as benzethonium will create swelling of the rER and damage the organelle. Ultimately, there is a loss in cell membrane integrity and cytosolic Ca2+ levels increase. Dysregulation of mitochondria and rER leads to the activation of caspase-2, caspase-8, caspase-9, and caspase-3.