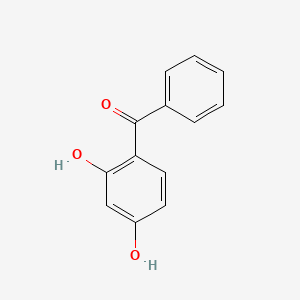

1. 2,4-dhb
2. Dhbp Cpd
1. 131-56-6
2. Benzophenone-1
3. (2,4-dihydroxyphenyl)(phenyl)methanone
4. Benzoresorcinol
5. Resbenzophenone
6. Inhibitor Dhbp
7. Methanone, (2,4-dihydroxyphenyl)phenyl-
8. Uvinul 400
9. Advastab 48
10. Uvistat 12
11. Uvinol 400
12. 4-benzoylresorcinol
13. Quinsorb 010
14. 4-benzoyl Resorcinol
15. Syntase 100
16. Dastib 263
17. Eastman Inhibitor Dhpb
18. Benzophenone, 2,4-dihydroxy-
19. Uf 1
20. Uv 12
21. Usaf Do-28
22. Usaf Nd-54
23. (2,4-dihydroxyphenyl)phenylmethanone
24. (2,4-dihydroxyphenyl)-phenyl-methanone
25. 2,4-dihydroxybenzofenon
26. Benzophenone 1
27. Nsc 38555
28. (2,4-dihydroxyphenyl)-phenylmethanone
29. (2,4-dihydroxy-phenyl)-phenyl-methanone
30. 2,4-dihydroxy Benzophenone
31. Mls000774789
32. [2,4-bis(oxidanyl)phenyl]-phenyl-methanone
33. Dtxsid8022406
34. Chebi:34240
35. Lj54r4z029
36. Nsc-38555
37. Hhb
38. Smr000365551
39. Benzophenone,4-dihydroxy-
40. Wln: Qr Cq Dvr
41. Dsstox_cid_2406
42. 2,4-dihydroxy-benzophenon
43. Dsstox_rid_76577
44. Dsstox_gsid_22406
45. Methanone,4-dihydroxyphenyl)phenyl-
46. 92092-63-2
47. Cas-131-56-6
48. Hsdb 5617
49. 2,4-dihydroxybenzofenon [czech]
50. Einecs 205-029-4
51. Brn 1311566
52. Unii-lj54r4z029
53. 2,4-dhb
54. Cut
55. Mfcd00002277
56. Eastman Inhibitor Dhbp
57. 2,4dihydroxybenzophenone
58. Enamine_001926
59. 4,6-dihydroxybenzophenone
60. 2,4-dihydroxy-benzophenone
61. 2,4-dihydroxybenzophe None
62. Ec 205-029-4
63. Cid_8572
64. Benzoresorcinol [mi]
65. Benzoresorcinolbenzoresorcinol
66. Chembl1392
67. 2,4-dihydroxybenzo- Phenone
68. Oprea1_840620
69. Schembl39681
70. Hsp90_163
71. Bidd:er0039
72. Benzophenone-1 [inci]
73. Uv-0
74. Bdbm51223
75. Nsc5358
76. 2,4-dihydroxybenzophenone, 99%
77. Hms1399h12
78. Hms2771p10
79. Zinc225430
80. Amy21954
81. Bcp25883
82. Nsc-5358
83. Nsc38555
84. Tox21_201285
85. Tox21_302865
86. Bbl013153
87. Stl163951
88. (2,4-dihydroxyphenyl)phenyl-methanon
89. Akos001019876
90. Ncgc00246026-01
91. Ncgc00246026-02
92. Ncgc00256606-01
93. Ncgc00258837-01
94. 2,4-dihydroxybenzophenone [hsdb]
95. Ac-11241
96. Ds-15473
97. D0573
98. Ft-0610114
99. 135d566
100. Ae-641/01968047
101. Q209209
102. Sr-01000388910
103. Q-200188
104. Sr-01000388910-1
| Molecular Weight | 214.22 g/mol |
|---|---|
| Molecular Formula | C13H10O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 214.062994177 g/mol |
| Monoisotopic Mass | 214.062994177 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Benzophenone-3 [2-hydroxy-4-methoxybenzophenone (HMB), oxybenzone, Spectra-Sorb UV-9 light absorber] is used in many cosmetics and sunscreens as a UV absorber. This study was conducted to investigate the metabolism of HMB (100 mg/kg body weight administered orally). 2,4-Dihydroxybenzophenone (DHB), 2,2'-dihydroxy-4-methoxybenzophenone (DHMB), and 2,3,4-trihydroxy-benzophenone (THB) metabolites were identified as free and conjugated forms by HPLC analysis. HMB was rapidly absorbed, metabolized, and detected in plasma (as free and protein bound) at 5 min postadministration. The parent compound and metabolites (free and conjugated) were detected at 6 hr in most tissues. DHB was present in most tissues with the highest concentration in the liver. DHMB was only detected as the conjugated form in liver, spleen, and heart. Trace amounts of THB were also detected in biological samples. Urine was the primary route, whereas feces was the secondary route of elimination of HMB and its metabolites. This study revealed O-dealkylation as the major pathway of HMB metabolism.
PMID:7902237 Okereke CS et al; Drug Metab Dispos 21 (5): 788-91 (1993)
Benzophenone-3 (2-hydroxy-4-methoxybenzophenone, BZ-3) is a UV absorber that is used extensively in medicine, cosmetics and industry as a sunscreen and color fastener. Exposure to the chemical is through the dermal and oral route. Bioavailability of the chemical absorbed through the skin is different from that seen through the oral route. The disposition of BZ-3 was investigated after dermal administration of 100 mg/kg body weight (body wt.) in Sprague-Dawley rats. Blood samples were collected at various intervals and the parent compound and its metabolites were analyzed by HPLC. Absorption was rapid as the parent compound and its metabolites were detected in plasma 5 min post-administration. The half-life (t1/2) of absorption was 3.45 hr corresponding to an absorption rate constant of 0.2 hr-1. Peak plasma concentration of 35 +/- 4.5 ug/mL (mean +/- standard error of the mean, S.E.) was attained at 2.5 hr post-administration. Disappearance from the plasma was biphasic with different half-lives (1.3 for alpha phase and 15.05 hr for beta phase), the area under the plasma concentration versus time curve was 211.1 +/- 38.2 ug/mL/hr (mean +/- S.E). There was also extensive binding of BZ-3 and its metabolites to plasma proteins. Three metabolites were identified in plasma, 2,4-dihydroxybenzophenone (DHB) and 2,2'-dihydroxy-4-methoxybenzophenone (DHMB) were the major metabolites detected in the plasma, while 2,3,4-trihydroxybenzophenone (THB) was detected in trace amounts. Tissue distribution studies revealed that THB was the major metabolite followed by DHB (both free and conjugated) in all tissues examined. The liver contained the highest amount followed by the kidney, spleen and testes, respectively.
PMID:8048080 Okereke CS et al; Toxicol Lett 73 (2): 113-22 (1994)
The metabolism and cytotoxicity of 2-hydroxy-4-methoxybenzophenone (HMB) in isolated rat hepatocytes and the xenoestrogenic activity of HMB and its metabolites in MCF-7 human breast cancer cells and an estrogen receptor competitive binding assay have been studied, respectively. The incubation of hepatocytes with HMB caused a concentration- and time-dependent decrease in cell viability, accompanied by loss of intracellular ATP and adenine nucleotide pools. HMB at a low-toxic level (0.25 mM) in the hepatocyte suspensions was converted enzymatically to 2,4-dihydroxybenzophenone (DHB) and a hydroxylated intermediate, which was tentatively identified as an isomer of 2,2prime prime or minute-dihydroxy-4-methoxybenzophenone (DHMB) as determined by mass spectroscopy coupled with HPLC. Furthermore, the parent compound and both intermediates were rapidly conjugated to glucuronides, whereas free unconjugated DHMB and 2,3,4-trihydroxybenzophenone (THB) were identified as trace intermediates. In another experiment, DHB and THB displaced competitively 17beta-estradiol bound to the recombinant human estrogen receptor alpha in a concentration-dependent manner: IC(50) of diethylstilbestrol and bisphenol A, which are known xenoestorogenic compounds, and DHB and THB was approximately 1 x 10(-8), 1 x 10(-5), 5 x 10(-5) and 5 x 10(-4) M, respectively. Further, DHB at concentrations from 10(-8) to 10(-6) M caused a concentration-dependent proliferation of MCF-7 cells. DHMB and THB at 10(-7) and 10(-6) M also elicited a slight increase in cell numbers, whereas HMB at concentrations from 10(-9) to 10(-4) M did not affect the cell proliferation. Based on the relative IC50 for the competitive binding and the proliferative effect on MCF-7 cells, it follows that in estrogenic potency, DHB>THB>DHMB. These results indicate that some hydroxylated intermediates such as DHB rather than the parent compound act as a xenoestrogen via biotransformation.
PMID:11823001 Nakagawa Y, Suzuki T; Chem Biol Interact 139 (2): 115-28 (2002)
Sunscreens containing UV filters are recommended to reduce damage caused by solar UV radiation. Recently, benzophenone (BP)-type UV filters have become widely used as UV stabilizers in skin-moisturizing products and sunscreen lotions; however, very little information is available regarding the potential harmful effects of prolonged exposure to these compounds. Therefore, we investigated the toxicokinetics and metabolism of BP-type UV filters in rats using gas chromatography-mass spectrometry (GC-MS). To examine the metabolism of BP-type UV filters, we analyzed the parent compounds BP and 2-hydroxy-4-methoxybenzophenone (HMB). In rats, BP was mainly converted to benzhydrol (BH) and 4-hydroxybenzophenone (HBP) (i.e., type A UV filters). In contrast, HMB was converted into at least three intermediates, including 2,4-dihydroxybenzophenone (DHB), which was formed via o-demethylation and subsequently converted into 2,3,4-trihydroxybenzophenone (THB), and 2,2'-dihydroxy-4-methoxybenzophenone (DHMB), which formed via the aromatic hydroxylation of HMB (i.e., type B UV filters). Next, the toxicokinetic curve for BP showed a peak concentration (Cmax) of 2.06+/-0.46 ug/mL at approximately 4h after BP administration. After a single oral dose of HMB, the Cmax of HMB reached 21.21+/-11.61 ug/mL within 3 hr (Tmax), and then declined rapidly compared to the kinetic curve of BP. The concentration of these metabolites in rat blood decreased much more slowly over time compared to the parent compounds. Thus, our results indicate that such metabolites might have more significant adverse effects than the parent compounds over the long term.
PMID:18448226 Jeon HK et al; Toxicology 248 (2-3): 89-95 (2008)
Benzophenone-3 (2-hydroxy-4-methoxybenzophenone, BZ-3) is a UV absorber that is used extensively in medicine, cosmetics and industry as a sunscreen and color fastener. Exposure to the chemical is through the dermal and oral route. Bioavailability of the chemical absorbed through the skin is different from that seen through the oral route. The disposition of BZ-3 was investigated after dermal administration of 100 mg/kg body weight (body wt.) in Sprague-Dawley rats. Blood samples were collected at various intervals and the parent compound and its metabolites were analyzed by HPLC. Absorption was rapid as the parent compound and its metabolites were detected in plasma 5 min post-administration. The half-life (t1/2) of absorption was 3.45 hr corresponding to an absorption rate constant of 0.2 hr-1. Peak plasma concentration of 35 +/- 4.5 ug/mL (mean +/- standard error of the mean, S.E.) was attained at 2.5 hr post-administration. Disappearance from the plasma was biphasic with different half-lives (1.3 for alpha phase and 15.05 hr for beta phase), the area under the plasma concentration versus time curve was 211.1 +/- 38.2 ug/mL/hr (mean +/- S.E). There was also extensive binding of BZ-3 and its metabolites to plasma proteins. Three metabolites were identified in plasma, 2,4-dihydroxybenzophenone (DHB) and 2,2'-dihydroxy-4-methoxybenzophenone (DHMB) were the major metabolites detected in the plasma, while 2,3,4-trihydroxybenzophenone (THB) was detected in trace amounts. Tissue distribution studies revealed that THB was the major metabolite followed by DHB (both free and conjugated) in all tissues examined. The liver contained the highest amount followed by the kidney, spleen and testes, respectively.
PMID:8048080 Okereke CS et al; Toxicol Lett 73 (2): 113-22 (1994)
When benzophenone-3 (2-hydroxy-4-methoxybenzophenone; BP-3) was incubated with liver microsomes of untreated rats in the presence of NADPH, the 5-hydroxylated metabolite, 2,5-dihydroxy-4-methoxybenzophenone (5-OH-BP-3), was formed as a major novel metabolite of BP-3. The 4-desmethylated metabolite, 2,4-dihydroxybenzophenone (2,4-diOH-BP), previously reported as the major in vivo metabolite of BP-3, was also detected. However, the amount of 5-OH-BP-3 formed in vitro was about the same as that of 2,4-diOH-BP. The oxidase activity affording 5-OH-BP-3 was inhibited by SKF 525-A and ketoconazole, and partly by quinidine and sulfaphenazole. The oxidase activity affording 2,4-diOH-BP was inhibited by SKF 525-A, ketoconazole and alpha-naphthoflavone, and partly by sulfaphenazole. The oxidase activity affording 5-OH-BP-3 was enhanced in liver microsomes of dexamethasone-, phenobarbital- and 3-methylcholanthrene-treated rats. The activity affording 2,4-diOH-BP was enhanced in liver microsomes of 3-methylcholanthrene- and phenobarbital-treated rats. When examined recombinant rat cytochrome P450 isoforms catalyzing the metabolism of BP-3, 5-hydroxylation was catalyzed by P450 3A2, 1A1, 2B1, 2C6 and 2D1, while 4-desmethylation was catalyzed by P450 2C6 and 1A1.
PMID:23190297 Kamikyouden N et al; Xenobiotica 43 (6): 514-9 (2013)
Epithelial-mesenchymal transition (EMT) is an important process in embryonic development and cancer progression and metastasis. EMT is influenced by 17beta-estradiol (E2), an endogenous estrogen. Benzophenone-1 (2,4-dihydroxybenzophenone, BP-1) and 4-tert-octylphenol (OP) are suspected endocrine disrupting chemicals (EDCs) because they can exhibit estrogenic properties. In this study, we examined whether BP-1 and OP can lead to EMT of BG-1 ovarian cancer cells expressing estrogen receptors (ERs). A wound healing assay and western blot assay were conducted to show the effect of BP-1 and OP on the migration of BG-1 cells and protein expression of EMT-related genes. BP-1 (10(-6) M) and OP (10(-6) M) significantly enhanced the migration capability of BG-1 cells by reducing the wounded area in the cell monolayer relative to the control, similar to E2 (10(-9) M). However, when BG-1 cells were co-treated with ICI 182,780, an ER antagonist, the uncovered area was maintained at the level of the control. N-cadherin, snail, and slug were increased by BP-1 and OP while E-cadherin was reduced compared to the control. However, this effect was also restored by co-treatment with ICI 182,780. Taken together, these results indicate that BP-1 and OP, the potential EDCs, may have the ability to induce ovarian cancer metastasis via regulation of the expression of EMT markers and migration of ER-expressing BG-1 ovarian cancer cells.
PMID:27145024 Shin S et al; Food Chem Toxicol 93: 58-65 (2016)
2,4-Dihydroxybenzophenone (benzophenone-1; BP-1) is an UV stabilizer primarily used to prevent polymer degradation and deterioration in quality due to UV irradiation. Recently, BP-1 has been reported to bioaccumulate in human bodies by absorption through the skin and has the potential to induce health problems including endocrine disruption. In the present study, we examined the xenoestrogenic effect of BP-1 on BG-1 human ovarian cancer cells expressing estrogen receptors (ERs) and relevant xenografted animal models in comparison with 17-beta estradiol (E2). In in vitro cell viability assay, BP-1 (10(-8)-10(-5)M) significantly increased BG-1 cell growth the way E2 did. The mechanism underlying the BG-1 cell proliferation was proved to be related with the up-regulation of cyclin D1, a cell cycle progressor, by E2 or BP-1. Both BP-1 and E2 induced cell growth and up-regulation of cyclin D1 were reversed by co-treatment with ICI 182,780, an ER antagonist, suggesting that BP-1 may mediate the cancer cell proliferation via an ER-dependent pathway like E2. On the other hand, the expression of p21, a regulator of cell cycle progression at G1 phase, was not altered by BP-1 though it was down-regulated by E2. In xenograft mouse models transplanted with BG-1 cells, BP-1 or E2 treatment significantly increased the tumor mass formation compared to a vehicle (corn oil) within 8 weeks. In histopathological analysis, the tumor sections of E2 or BP-1 group displayed extensive cell formations with high density and disordered arrangement, which were supported by the increased number of BrdUrd positive nuclei and the over-expression of cyclin D1 protein. Taken together, these results suggest that BP-1 is an endocrine disrupting chemical (EDC) that exerts xenoestrogenic effects by stimulating the proliferation of BG-1 ovarian cancer via ER signaling pathway associated with cell cycle as did E2.
PMID:23328252 Park MA et al; Toxicology 305: 41-8 (2013)
Prostate cancer (PCa) is a global health concern in human males. Recently, it has been known that endocrine-disrupting chemicals (EDCs) may act as an exogenous factor to enhance cancer progression. Triclosan (TCS) and 2,4-dihydroxybenzophenone (BP-1) were reported to bioaccumulate in human bodies through the skin absorption. However, there has been insufficient evidence on the findings that the intervention of EDCs may promote the cancer progression in PCa. In the present study, to verify the risk of TCS and BP-1 to a PCa progression, cancer cell proliferation and migration were investigated in LNCaP PCa cells. TCS and BP-1 increased LNCaP cell proliferative activity and migration as did dihydrotestosterone (DHT). This phenomenon was reversed by the treatment with bicalutamide, a well known androgen receptor (AR) antagonist, suggesting that TCS and BP-1 acted as a xenoandrogen in LNCaP cells via AR signaling pathway by mimicking the action of DHT. A Western blot assay was performed to identify the alterations in the translational levels of cell growth- and metastasis-related markers, i.e., c-fos, cyclin E, p21, and cathepsin D genes. The expressions of genes related with G1/S transition of cell cycle and metastasis were increased by DHT, TCS, and BP-1, while the expression of p21 protein responsible for cell cycle arrest was reduced by DHT, TCS, and BP-1. Taken together, these results indicated that TCS and BP-1 may enhance the progression of PCa by regulating cell cycle and metastasis-related genes via AR signaling pathway.
PMID:25682003 Kim SH et al; Environ Toxicol Pharmacol 39 (2): 568-76 (2015)