


1. Rta744
2. Wp 744
3. Wp-744
4. Wp744 Cpd
1. Berubicin Hcl
2. Wp744
3. Berubicin Hydrochloride [usan]
4. Rta 744
5. Wp-744
6. 293736-67-1
7. Rta-744
8. 7ba3x03948
9. Berubicin Hydrochloride (usan)
10. Unii-7ba3x03948
11. Wp 744
12. Chembl2103796
13. Dtxsid10951913
14. Berubicin Hydrochloride [who-dd]
15. 293736-67-1 (hcl)
16. D08871
17. Q27268015
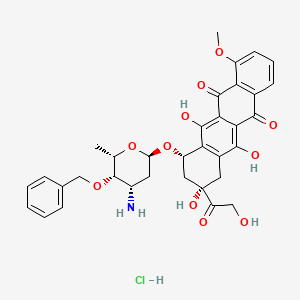
18. (7s,9s)-7-[(2r,4s,5s,6s)-4-amino-6-methyl-5-phenylmethoxyoxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydrochloride
19. (8s,10s)-10-(((2r,4s,5s,6s)-4-amino-5-(benzyloxy)-6-methyltetrahydro-2h-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Hydrochloride
20. (8s,10s)-10-(((2r,4s,5s,6s)-4-amino-5-(benzyloxy)-6-methyltetrahydro-2h-pyran-2-yl)oxy)-8-glycoloyl-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Hydrochloride
21. (8s,10s)-10-((3-amino-4-o-benzyl-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)- 6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene- 5,12-dione Hydrochloride
22. 3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-4-o-benzyl-2,3,6-trideoxyhexopyranoside--hydrogen Chloride (1/1)
23. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-4-o-(phenylmethyl)-.alpha.-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8s,10s)-, Hydrochloride
24. 5,12-naphthacenedione, 10-((3-amino-2,3,6-trideoxy-4-o-(phenylmethyl)-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, Hydrochloride, (8s,10s)-
| Molecular Weight | 670.1 g/mol |
|---|---|
| Molecular Formula | C34H36ClNO11 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 669.1976887 g/mol |
| Monoisotopic Mass | 669.1976887 g/mol |
| Topological Polar Surface Area | 195 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 1130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Investigated for use/treatment in brain cancer.
RTA 744 is a substance being studied in the treatment of adult brain tumors. RTA 744 crosses the blood-brain barrier and blocks an enzyme needed for cancer growth. RTA 744 is a type of topoisomerase inhibitor. Also called topoisomerase II inhibitor RTA 744.