

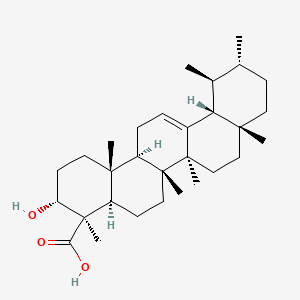

1. Boswellic Acid
1. 631-69-6
2. B-boswellic Acid
3. (3r,4r,4ar,6ar,6bs,8ar,11r,12s,12ar,14ar,14br)-3-hydroxy-4,6a,6b,8a,11,12,14b-heptamethyl-2,3,4a,5,6,7,8,9,10,11,12,12a,14,14a-tetradecahydro-1h-picene-4-carboxylic Acid
4. B252m1yo2v
5. Chembl267225
6. (4r)-3alpha-hydroxyurs-12-en-24-oic Acid
7. Unii-b252m1yo2v
8. Mfcd04039448
9. Urs-12-en-24-oic Acid, 3.alpha.-hydroxy-
10. Mls000697685
11. .beta.-boswellic Acid
12. Schembl4385785
13. Dtxsid2057578
14. Urs-12-en-23-oic Acid, 3-hydroxy-, (3alpha,4beta)-
15. Beta-boswellic Acid, Hplc Grade
16. Chebi:192014
17. Urs-12-en-23-oic Acid, 3-hydroxy-, (3.alpha.,4.beta.)-
18. 3-hydroxy-12-ursen-24-oic Acid
19. (3r,4r,4ar,6ar,6bs,8ar,11r,12s,12ar,14ar,14br)-3-hydroxy-4,6a,6b,8a,11,12,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicene-4-carboxylic Acid
20. Hy-n2513
21. .beta.-boswellic Acid [mi]
22. Bdbm50241260
23. Zinc14089743
24. Akos015897120
25. Lmpr0106180011
26. Beta-boswellic Acid, Analytical Standard
27. Ncgc00247633-01
28. Ac-30252
29. Smr000445587
30. Boswellia Serrata Hexane Extract In Hexane
31. Cs-0022784
32. (3alpha,4beta)-3-hydroxy-urs-12-en-23-oate
33. 3.alpha.-hydroxyurs-12-en-24-oic Acid
34. 631b696
35. (3alpha,4beta)-3-hydroxy-urs-12-en-23-oic Acid
36. Q27274271
37. .beta.-boswellic Acid (constituent Of Boswellia Serrata) [dsc]
| Molecular Weight | 456.7 g/mol |
|---|---|
| Molecular Formula | C30H48O3 |
| XLogP3 | 8.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 456.36034539 g/mol |
| Monoisotopic Mass | 456.36034539 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 878 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)