

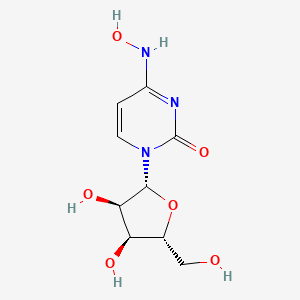

1. 4-oxime Uridine
2. Beta-d-n4-hydroxycytidine
3. Eidd-1931
4. N4-hydroxycytidine
1. N4-hydroxycytidine
2. Eidd-1931
3. Beta-d-n4-hydroxycytidine
4. N-hydroxycytidine
5. 3258-02-4
6. 4-n-hydroxycytidine
7. C3d11pv2o4
8. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-(hydroxyamino)pyrimidin-2-one
9. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxyimino)-3,4-dihydropyrimidin-2(1h)-one
10. 4-oxime Uridine
11. N-4-hydroxycytidine
12. Cytidine, N-hydroxy-
13. Ccris 1074
14. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-4-(hydroxyimino)-3,4-dihydropyrimidin-2(1h)-one
15. Unii-c3d11pv2o4
16. Eidd1931
17. Beta-d-n(4)-hydroxycytidine
18. N-hydroxy-3,4-dihydrocytidine
19. Schembl5190819
20. Chembl2178720
21. Eidd 1931
22. Gtpl10735
23. Dtxsid20186274
24. Chebi:180654
25. Ex-a3655
26. Mfcd01675695
27. At13085
28. Bs-47105
29. Hy-125033
30. Cs-0088698
31. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxyamino)pyrimidin-2(1h)-one
32. 1-((2r,3r,4s,5r)-3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-4-hydroxyamino-1h-pyrimidin-2-one
33. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-(hydroxyamino)-1,2-dihydropyrimidin-2-one
34. 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-4-(hydroxyamino)pyrimidin-2-one
| Molecular Weight | 259.22 g/mol |
|---|---|
| Molecular Formula | C9H13N3O6 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 259.08043514 g/mol |
| Monoisotopic Mass | 259.08043514 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 398 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
N4-hydroxycytidine and its prodrug [EIDD-2801] is being studied for its activity against a number of viral infections including influenza, MERS-CoV, and SARS-CoV-2.
Absorption
N4-hydroxycytidine is orally bioavailable in mice but poorly bioavailable in non-human primates.
N4-hydroxycytidine distributes into tissues where it is is phosphorylated to the 5'-triphosphate form.
N4-hydroxycytidine is phosphorylated in tissue to the active 5-triphosphate form, which is incorporated into the genome of new virions, resulting in the accumulation of inactivating mutations, known as viral error catastrophe. A [remdesivir] resistant mutant mouse hepatitis virus has also been shown to have increased sensitivity to N4-hydroxycytidine.