
1. Aequamen
2. Betahistin Al
3. Betahistin Ratiopharm
4. Betahistin Stada
5. Betahistin-ratiopharm
6. Betahistine
7. Betahistine Biphar
8. Betahistine Dihydrobromide
9. Betahistine Dihydrochloride
10. Betahistine Hydrochloride
11. Betahistine Mesylate
12. Betahistine Methanesulfonate
13. Betahistine Methanesulphonate
14. Betaserc
15. Betavert
16. By Vertin
17. By-vertin
18. Dihydrobromide, Betahistine
19. Dihydrochloride, Betahistine
20. Extovyl
21. Fidium
22. Hydrochloride, Betahistine
23. Lectil
24. Melopat
25. Mersilon
26. Mesylate, Betahistine
27. Methanesulfonate, Betahistine
28. Methanesulphonate, Betahistine
29. Pt 9
30. Pt-9
31. Pt9
32. Ribrain
33. Serc
34. Vasomotal
35. Vertigon
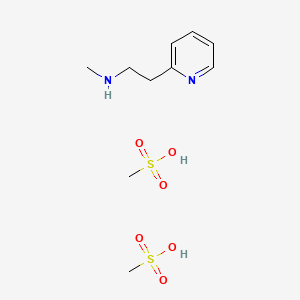
1. Betahistine Mesylate
2. 54856-23-4
3. Meginalisk
4. Merislon
5. Extovyl
6. Melopat
7. 2-pyridineethanamine, N-methyl-, Dimethanesulfonate
8. Tenyl
9. Betahistine Methanesulfonate
10. Betahistine Dimesylate
11. Betahistine (mesylate)
12. Methanesulfonic Acid;n-methyl-2-pyridin-2-ylethanamine
13. X1l0e3r43y
14. 2-[2-(methylamino)ethyl]pyridine Methanesulfonate
15. Riptonin
16. Einecs 259-377-7
17. Unii-x1l0e3r43y
18. Tenoxican
19. Suzutolon (tn)
20. 2-(ethylammonio)-n-methylpyridinium Dimethanesulphonate
21. Betahistine Dimethanesulfonate
22. Betahistine Mesilate (jp17)
23. Betahistine Di Mesylate
24. Schembl194604
25. Chembl4303472
26. Chebi:31274
27. Hms2096n07
28. Hms3713n07
29. Hms3886k08
30. Bcp13129
31. Hy-d0237
32. Betahistine Mesilate [mart.]
33. Mfcd00211321
34. S5498
35. Betahistine Mesilate [who-dd]
36. Akos015898737
37. Ccg-220543
38. Ac-23916
39. Betahistine Mesilate [ep Impurity]
40. Betahistine Mesilate [ep Monograph]
41. Db-052657
42. Cs-0010146
43. Ft-0622912
44. D01592
45. D87714
46. N-methyl-2-pyridineethylamine Dimethanesulfonate
47. Bethahistinemethanesulfonate;betahistine Mesilate
48. N-methyl-2-(2-pyridinyl)ethanamine Methanesulfonate
49. Sr-01000722006-3
50. W-105601
51. Q27293318
52. N-methyl-2-(pyridin-2-yl)ethanamine Dimethanesulfonate
53. 2-pyridineethanamine, N-methyl-, Dimethanesulphonate
54. N-methyl-2-(pyridin-2-yl)ethan-1-amine Dimethanesulfonate
55. 2-pyridineethanamine, N-methyl-, Methanesulfonate (1:2)
56. 2-pyridineethanamine, N-methyl-, Methanesulphonate (1:2)
| Molecular Weight | 328.4 g/mol |
|---|---|
| Molecular Formula | C10H20N2O6S2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 328.07627871 g/mol |
| Monoisotopic Mass | 328.07627871 g/mol |
| Topological Polar Surface Area | 150 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 176 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Histamine Agonists
Drugs that bind to and activate histamine receptors. Although they have been suggested for a variety of clinical applications histamine agonists have so far been more widely used in research than therapeutically. (See all compounds classified as Histamine Agonists.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)

LOOKING FOR A SUPPLIER?