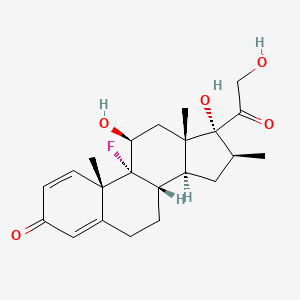

1. Betadexamethasone
2. Celeston
3. Celestona
4. Celestone
5. Cellestoderm
6. Flubenisolone
1. 378-44-9
2. Betadexamethasone
3. Flubenisolone
4. Celestene
5. Rinderon
6. Visubeta
7. Betamethazone
8. Becort
9. Desacort-beta
10. Betacorlan
11. Betacortril
12. Betamamallet
13. Betametasone
14. Betapredol
15. Betasolon
16. Betnelan
17. Betsolan
18. Methazon
19. Bedifos
20. Cidoten
21. Celestone
22. Beta-methasone
23. Rinderon A
24. Beta-methasone Alcohol
25. Betametasona
26. Betamethasonum
27. Sch 4831
28. Bebate
29. 9alpha-fluoro-16beta-methylprednisolone
30. Nsc-39470
31. 9-fluoro-16beta-methylprednisolone
32. Sch-4831
33. Betamethasone (celestone)
34. 9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione
35. Betamethasone Cream
36. Mls000859943
37. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11.beta.,16.beta.)-
38. Chebi:3077
39. Betamethasone Alcohol
40. Betametasone [dcit]
41. Ncs-39470
42. 9842x06q6m
43. (8s,9r,10s,11s,13s,14s,16s,17r)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
44. Ncgc00164401-01
45. Smr000058601
46. Celestone Syrup And Tablets
47. Betametasona [inn-spanish]
48. Betamethasonum [inn-latin]
49. Dsstox_cid_2667
50. 9a-fluoro-11b,17a,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione
51. 16beta-methyl-1,4-pregnadiene-9alpha-fluoro-11beta,17alpha,21-triol-3,20-dione
52. Dsstox_rid_76681
53. Dsstox_gsid_22667
54. (8s,9r,10s,11s,13s,14s,16s,17r)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
55. .beta.-methasone
56. 9-fluoro-16.beta.-methylprednisolone
57. (11beta,16beta)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
58. Rinderon (tn)
59. .beta.-methasone Alcohol
60. 9.alpha.-fluoro-16.beta.-methylprednisolone
61. 9-alpha-fluoro-16-beta-methylprednisolone
62. Hsdb 3015
63. Einecs 206-825-4
64. Mfcd00062969
65. Nsc 39470
66. Prednisolone, 9-fluoro-16beta-methyl-
67. Brn 3176546
68. Nsc39470
69. Unii-9842x06q6m
70. Desacort-.beta.
71. .beta.-corlan
72. 9alpha-fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-1,4-pregnadiene-3,20-dione
73. Ncgc00091019-08
74. Cas-378-44-9
75. Prestwick_703
76. Prednisolone, 9-fluoro-16.beta.-methyl-
77. 9-fluoro-11-beta,17,21-trihydroxy-16-beta-methylpregna-1,4-diene-3,20-dione
78. Flosteron
79. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11beta,16beta)-
80. Betamethasone, Topical
81. Betamethasone [usan:usp:inn:ban:jan]
82. Corticosterone, 1-dehydro-9-fluoro-17-hydroxy-16beta-methyl-
83. Betamethasone, >=98%
84. Prestwick0_000362
85. Prestwick1_000362
86. Prestwick2_000362
87. Prestwick3_000362
88. Betamethasone [mi]
89. Chembl632
90. Betamethasone [inn]
91. Betamethasone [jan]
92. Ec 206-825-4
93. Cid_9782
94. Schembl4565
95. 16-beta-methyl-1,4-pregnadiene-9-alpha-fluoro-11-beta,17-alpha,21-triol-3,20-dione
96. 9-alpha-fluoro-16-beta-methyl-1,4-pregnadiene-11-beta,17-alpha,21-triol-3,20-dione
97. Betamethasone [usan]
98. Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17,21-trihydroxy-16beta-methyl-
99. Bidd:pxr0047
100. Betamethasone [vandf]
101. Bspbio_000483
102. 4-08-00-03501 (beilstein Handbook Reference)
103. Mls001066413
104. Mls001332616
105. Mls002153244
106. Betamethasone [mart.]
107. Spbio_002404
108. Betamethasone [usp-rs]
109. Betamethasone [who-dd]
110. Betamethasone [who-ip]
111. Corticosterone, 1-dehydro-9-fluoro-17-hydroxy-16.beta.-methyl-
112. Bpbio1_000533
113. Gtpl7061
114. Dtxsid3022667
115. Bdbm73823
116. Betamethasone (jp17/usp/inn)
117. Bcpp000345
118. Hms1569i05
119. Hms2096i05
120. Hms2233i08
121. Hms3713i05
122. Betamethasone [ep Impurity]
123. Betamethasone [orange Book]
124. Bcp02020
125. Zinc3876136
126. Betamethasone [ep Monograph]
127. Tox21_112115
128. Tox21_301455
129. Betamethasone [usp Monograph]
130. S1500
131. Betamethasonum [who-ip Latin]
132. Akos008901360
133. Akos015894863
134. Tox21_112115_1
135. Bcp9000393
136. Ccg-220362
137. Cs-1897
138. Db00443
139. Ks-5302
140. 9-fluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione
141. Smp1_000043
142. Betamethasone Dipropionate Ep Impurity A
143. Ncgc00164401-02
144. Ncgc00164401-03
145. Ncgc00255195-01
146. Hy-13570
147. Betamethasone 1000 Microg/ml In Methanol
148. Betamethasone Dipropionate Impurity A
149. Betamethasone 100 Microg/ml In Acetonitrile
150. D1961
151. Betamethasone 1000 Microg/ml In Acetonitrile
152. Dexamethasone Impurity B [ep Impurity]
153. Betamethasone, Meets Usp Testing Specifications
154. C06848
155. D00244
156. D88866
157. 378m449
158. Betamethasone, Vetranal(tm), Analytical Standard
159. Q416132
160. Sr-01000780582
161. Sr-01000780582-2
162. W-106509
163. Betamethasone Acetate Impurity A [ep Impurity]
164. Brd-k39188321-001-03-9
165. Betamethasone, British Pharmacopoeia (bp) Reference Standard
166. Betamethasone, European Pharmacopoeia (ep) Reference Standard
167. Betamethasone, Pharmaceutical Impurity Standard, >=95.0% (hplc)
168. Betamethasone, United States Pharmacopeia (usp) Reference Standard
169. (11.beta.,16.beta.)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
170. 9-.alpha.-fluoro-11-.beta.,17,21-trihydroxy-16-.beta.-methylpregna-1,4-diene-3,20-dione
171. 9-fluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione.
172. 9-fluoro-16beta-methyl-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione
173. Betamethasone, Pharmaceutical Secondary Standard; Certified Reference Material
174. Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17,21-trihydroxy-16.beta.-methyl-
175. Pregna-1,4-diene-3,20-dione, 9alpha-fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-
176. (1r,2s,10s,11s,13s,14r,15s,17s)-1-fluoro-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,13,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
177. 16-.beta.-methyl-1,4-pregnadiene-9-.alpha.-fluoro-11-.beta.,17-.alpha.,21-triol-3,20-dione
178. 9-.alpha.-fluoro-11-.beta.,17-.alpha.,21-trihydroxy-16-.beta.-methylpregna-1,4-diene-3,20-dione
179. 9-.alpha.-fluoro-16-.beta.-methyl-1,4-pregnadiene-11-.beta.,17-.alpha.,21-triol-3,20-dione
180. 9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione, (11.beta.,16.beta.) #
181. Betamethasone; 9-fluoro-11?,17,21-trihydroxy-16?-methylpregna-1,4-diene-3,20-dione; (11?,16?)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 9?-fluoro-16?-methylprednisolone
1. Betamethasone Butyrate Propionate
| Molecular Weight | 392.5 g/mol |
|---|---|
| Molecular Formula | C22H29FO5 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 392.19990218 g/mol |
| Monoisotopic Mass | 392.19990218 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 805 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Lotrisone |
| PubMed Health | Betamethasone/Clotrimazole (On the skin) |
| Drug Classes | Anti-Infective/Anti-Inflammatory Combination, Antibacterial Combination |
| Active Ingredient | clotrimazole; Betamethasone dipropionate |
| Dosage Form | Cream; Lotion |
| Route | Topical |
| Strength | eq 0.05% base; 1% |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 2 of 4 | |
|---|---|
| Drug Name | Luxiq |
| PubMed Health | Betamethasone |
| Drug Classes | Corticosteroid, Intermediate, Corticosteroid, Strong, Corticosteroid, Very Strong, Diagnostic Agent, Adrenocortical Function, Endocrine-Metabolic Agent, Immune Suppressant |
| Active Ingredient | Betamethasone valerate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.12% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 3 of 4 | |
|---|---|
| Drug Name | Lotrisone |
| PubMed Health | Betamethasone/Clotrimazole (On the skin) |
| Drug Classes | Anti-Infective/Anti-Inflammatory Combination, Antibacterial Combination |
| Active Ingredient | clotrimazole; Betamethasone dipropionate |
| Dosage Form | Cream; Lotion |
| Route | Topical |
| Strength | eq 0.05% base; 1% |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 4 of 4 | |
|---|---|
| Drug Name | Luxiq |
| PubMed Health | Betamethasone |
| Drug Classes | Corticosteroid, Intermediate, Corticosteroid, Strong, Corticosteroid, Very Strong, Diagnostic Agent, Adrenocortical Function, Endocrine-Metabolic Agent, Immune Suppressant |
| Active Ingredient | Betamethasone valerate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.12% |
| Market Status | Prescription |
| Company | Delcor Asset |
Anti-Asthmatic Agents; Anti-Inflammatory Agents, Steroidal; Glucocorticoids, Synthetic; Glucocorticoids, Topical
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Indicated for the treament of/ allergic disorders: drug induced allergic reactions; angioedema; acute noninfectious laryngeal edema; allergic, perennial or seasonal, severe rhinitis; serum sickness; urticarial transfusions reactions.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1002
/Indicated for the treament of/ collagen disorders: acute, rheumatic or nonrheumatic carditis; systemic lupus erythematosus; mixed connective tissue disease; polyarteritis nodosa; relapsing polychondritis.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1002
/Indicated for the treatment of/ dermatologic disorders: alopecia areata; atopic dermatitis; contact dermatitis; exfoliative dermatitis; herpetiformis, bullous dermatitis; severe, seborrheic dermatitis; severe inflammatory dermatoses; severe multiforme erythema; granuloma annulare; keloids; lichen planus; lichen simplex chronicus; discoid lupus erythematosus; mycosis fungoides; necrobiosis lipoidica diabeticorum; pemphigus; severe psoriasis; psoriatic plaques; severa eczema; pemphigoid; localized cutaneous sarcoid.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1003
For more Therapeutic Uses (Complete) data for BETAMETHASONE (16 total), please visit the HSDB record page.
... The most striking effects of corticosteroids on the cardiovascular system result from mineralocorticoid-induced changes in renal Na + excretion as is evident in primary aldosteronism. The resultant hypertension can lead to a diverse group of adverse effects on the cardiovascular system, including increased atherosclerosis, cerebral hemorrhage, stroke, and hypertensive cardiomyopathy. The mechanism underlying the hypertension remains incompletely understood, but restriction of dietary Na + can lower the blood pressure considerably. /Adrenocorticosteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1661
Two effects of corticosteroids on lipid metabolism are firmly established. The first is the dramatic redistribution of body fat that occurs in settings of hypercorticism such as Cushing's syndrome. The other is the permissive facilitation of the effect of other agents, such as growth hormone and beta-adrenergic receptor agonists, in inducing lipolysis in adipocytes, with a resultant increase in free fatty acids following glucocorticoid administration. /Adrenocorticosteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1659
...Caution should be exercised if fluorinated preparations are used on face or other cosmetically important areas, since paradoxical eruptions may occur with long-term use.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 513
Although injection may be given intra-articularly, it must be remembered that repeated intra-articular glucocorticoids sometimes effect painless destruction of joint.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 889
For more Drug Warnings (Complete) data for BETAMETHASONE (34 total), please visit the HSDB record page.
As a member of the corticosteroid family, betamethasone is indicated for the treatment of several inflammatory conditions. As topical monotherapy, betamethasone is indicated to relieve pruritic and inflammatory symptoms of corticosteroid-responsive-dermatoses. Betamethasone can be used topically in combination with a vitamin D analog such as calcipotriene to treat plaque psoriasis. The corticosteroid is also available as an injectable suspension and can be used to manage a range of inflammatory conditions including endocrine disorders, gastrointestinal disorders, and rheumatic disorders among other conditions.
FDA Label
Corticosteroids bind to the glucocorticoid receptor inhibiting pro-inflammatory signals, while promoting anti-inflammatory signals. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients who require long-term treatment with a corticosteroid should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Asthmatic Agents
Drugs that are used to treat asthma. (See all compounds classified as Anti-Asthmatic Agents.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D07AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EA - Corticosteroids acting locally
A07EA04 - Betamethasone
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA05 - Betamethasone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC01 - Betamethasone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XC - Corticosteroids, potent, other combinations
D07XC01 - Betamethasone
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02A - Corticosteroids for systemic use, plain
H02AB - Glucocorticoids
H02AB01 - Betamethasone
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AD - Corticosteroids
R01AD06 - Betamethasone
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BA - Glucocorticoids
R03BA04 - Betamethasone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA06 - Betamethasone
S - Sensory organs
S01 - Ophthalmologicals
S01C - Antiinflammatory agents and antiinfectives in combination
S01CB - Corticosteroids/antiinfectives/mydriatics in combination
S01CB04 - Betamethasone
S - Sensory organs
S02 - Otologicals
S02B - Corticosteroids
S02BA - Corticosteroids
S02BA07 - Betamethasone
S - Sensory organs
S03 - Ophthalmological and otological preparations
S03B - Corticosteroids
S03BA - Corticosteroids
S03BA03 - Betamethasone
Absorption
The absorption and potency of any topical corticosteroid including betamethasone depends on the vehicle in which the steroid is delivered. For example, betamethasone dipropionate 0.05% ointment is classified as a highly potent topical steroid, while betamethasone dipropionate 0.05% cream or lotion is considered to be moderately potent. There are several structural modifications that can determine the potency of a topical corticosteroid. For example, corticosteroids containing a halogen at specific carbons, or that contain esters are more potent due to enhanced lipophilicity. As such, there is a marked difference between topical products containing betamethasone dipropionate vs. betamethasone valerate. Betamethasone dipropionate contains 2 esters which enhances its potency, while betamethasone valerate has only one ester and is less potent. It should be noted that the use of occlusive dressings with topical steroids significantly increases the absorption, increasing the risk for adverse effects.
Route of Elimination
Corticosteroids are eliminated predominantly in the urine.
Volume of Distribution
In a study that included Indian women of reproductive age, the volume of distribution following a single intramuscular dose of betamethasone phosphate was 94,58423,539 mL(s).
Clearance
In a study that included Indian women of reproductive age, the CL/F following a single intramuscular dose of betamethasone phosphate was 6,466 805 mL/hour.
Glucocorticoids ... absorbed systemically from sites of local administration, such as synovial spaces, the conjunctival sac, skin, and respiratory tract. When administration is prolonged, when the site of application is covered with an occlusive dressing, or when large areas of skin are involved, the absorption may be sufficient to cause systemic effects, including suppression of the HPA axis. /Adrenocorticalsteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1663
Following absorption, 90% or more of cortisol in plasma is reversibly bound to protein under normal circumstances. Only the fraction of corticosteroid that is unbound can enter cells to mediate corticosteroid effects. Two plasma proteins account for almost all of the steroid-binding capacity: corticosteroid-binding globulin (CBG; also called transcortin), and albumin. CBG is an alpha-globulin secreted by the liver that has high affinity for steroids but relatively low total binding capacity, whereas albumin, also produced by the liver, has low affinity but relatively large binding capacity. At normal or low concentrations of corticosteroids, most of the hormone is protein-bound. At higher steroid concentrations, the capacity of protein binding is exceeded, and a significantly greater fraction of the steroid exists in the free state. Corticosteroids compete with each other for binding sites on CBG. CBG has relatively high affinity for cortisol and most of its synthetic congeners and low affinity for aldosterone and glucuronide-conjugated steroid metabolites; thus, greater percentages of these latter steroids are found in the free form. /Adrenocortical Steroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1663
The pharmacokinetics of betamethasone and its phosphate ester are described in 8 healthy adults after iv bolus injection of 10.6 mg betamethasone phosphate. Both cmpd were measured by high performance liquid chromatography with ultraviolet detection using sample handling methods which prevented hydrolysis of the ester in vitro. Betamethasone phosphate disappeared rapidly from plasma (mean half-life = 4.7 min) as betamethasone levels rose. Betamethasone plasma levels reached a peak 10-36 min after admin of the phosphate before declining in a biexponential manner. The terminal slow disposition phase had a mean half-life of 6.5 hr. Only about 5% of the dose was recovered from urine as betamethasone, indicating extensive extrarenal clearance of betamethasone. /Betamethason phosphate/
PMID:6662164 Petersen MC, et al; Eur J Clin Pharmacol 25 (5): 643-50 (1983)
The metabolism of betamethasone yields 6 metabolites. The metabolic processes include 6 hydroxylation, 11-hydroxyl oxidation, and reduction of the C-20 carbonyl group followed by removal of the side chain.
All of the biologically active adrenocortical steroids and their synthetic congeners have a double bond in the 4,5 position and a ketone group at C 3. As a general rule, the metabolism of steroid hormones involves sequential additions of oxygen or hydrogen atoms, followed by conjugation to form water-soluble derivatives. Reduction of the 4,5 double bond occurs at both hepatic and extrahepatic sites, yielding inactive compounds. Subsequent reduction of the 3-ketone substituent to the 3-hydroxyl derivative, forming tetrahydrocortisol, occurs only in the liver. Most of these A ring-reduced steroids are conjugated through the 3-hydroxyl group with sulfate or glucuronide by enzymatic reactions that take place in the liver and, to a lesser extent, in the kidney. The resultant sulfate esters and glucuronides form water-soluble derivatives and are the predominant forms excreted in the urine. Neither biliary nor fecal excretion is of quantitative importance in human beings. /Adrenocortical Steroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1663
In a study that included Indian women of reproductive age, the half-life following a single intramuscular dose of betamethasone phosphate was 10.2 2.5 hours.
The pharmacokinetics of betamethasone and its phosphate ester are described in 8 healthy adults after i.v. bolus injection of 10.6 mg betamethasone phosphate. Both compounds were measured by high-performance liquid chromatography with ultraviolet detection using sample handling methods which prevented hydrolysis of the ester in vitro. Betamethasone phosphate disappeared rapidly from plasma (mean half-life = 4.7 min) as betamethasone levels rose. Betamethasone plasma levels reached a peak 10-36 min after administration of the phosphate before declining in a biexponential manner. The terminal slow disposition phase had a mean half-life of 6.5 hr.
PMID:6662164 Petersen M et al; Eur J Clin Pharmacol 25 (5): 643-50 (1983)
Serum half-life of betamethasone is about 3 hr.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 889
Glucocorticoids inhibit neutrophil apoptosis and demargination, and inhibit NF-Kappa B and other inflammatory transcription factors. They also inhibit phospholipase A2, leading to decreased formation of arachidonic acid derivatives. In addition, glucocorticoids promote anti-inflammatory genes like interleukin-10. Corticosteroids like betamethasone can act through nongenomic and genomic pathways. The genomic pathway is slower and occurs when glucocorticoids activate glucocorticoid receptors and initiate downstream effects that promote transcription of anti-inflammatory genes including phosphoenolpyruvate carboxykinase (PEPCK), IL-1-receptor antagonist, and tyrosine amino transferase (TAT). On the other hand, the nongenomic pathway is able to elicit a quicker response by modulating T-cell, platelet and monocyte activity through the use of existing membrane-bound receptors and second messengers.
Corticosteroids interact with specific receptor proteins in target tissues to regulate the expression of corticosteroid responsive genes, thereby changing the levels and array of proteins synthesized by the various target tissues. As a consequence of the time required for changes in gene expression and protein synthesis, most effects of corticosteroids are not immediate, but become apparent after several hours. ... Although corticosteroids predominantly act to increase expression of target genes, there are well documented examples where glucocorticoids decrease transcription of target genes ... In contrast to these genomic effects, recent studies have raised the possibility that some actions of corticosteroids are immediate and are mediated by membrane-bound receptors. /Adrenocorticosteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1657
The mechanisms by which glucocorticoids inhibit glucose utilization in peripheral tissues are not fully understood. Glucocorticoids decrease glucose uptake in adipose tissue, skin, fibroblasts, thymocytes, and polymorphonuclear leukocytes; these effects are postulated to result from translocation of the glucose transporters from the plasma membrane to an intracellular location. These peripheral effects are associated with a number of catabolic actions, including atrophy of lymphoid tissue, decreased muscle mass, negative nitrogen balance, and thinning of the skin. /Adrenocorticalsteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1659
The mechanisms by which the glucocorticoids promote gluconeogenesis are not fully defined. Amino acids mobilized from a number of tissues in response to glucocorticoids reach the liver and provide substrate for the production of glucose and glycogen. In the liver, glucocorticoids induce the transcription of a number of enzymes involved in gluconeogenesis and amino acid metabolism, including phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, and fructose-2,6-bisphosphatase. Analyses of the molecular basis for regulation of phosphoenolpyruvate carboxykinase gene expression have identified complex regulatory influences involving an interplay among glucocorticoids, insulin, glucagon, and catecholamine. The effects of these hormones and amines on phosphoenolpyruvate carboxykinase gene expression mirror the complex regulation of gluconeogenesis in the intact organism. /Adrenocorticalsteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1659
... /A/ major action of corticosteroids on the cardiovascular system is to enhance vascular reactivity to other vasoactive substances. Hypoadrenalism generally is associated with hypotension and reduced response to vasoconstrictors such as norepinephrine and angiotensin II. This diminished pressor response is explained partly by recent studies in experimental systems showing that glucocorticoids increase expression of adrenergic receptors in the vascular wall. Conversely, hypertension is seen in patients with excessive glucocorticoid secretion, occurring in most patients with Cushing's syndrome and in a subset of patients treated with synthetic glucocorticoids (even those lacking any significant mineralocorticoid action). /Adrenocorticosteroids/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1660
For more Mechanism of Action (Complete) data for BETAMETHASONE (8 total), please visit the HSDB record page.