


1. Bethanechol
2. Bethanecol
3. Chloride, Bethanechol
4. Duvoid
5. Hermes, Myo
6. Myo Hermes
7. Myocholine
8. Myotonachol
9. Myotonine
10. Pms Bethanechol Chloride
11. Pms-bethanechol Chloride
12. Urecholine
13. Urocarb
1. 590-63-6
2. Urecholine
3. Duvoid
4. Myocholine
5. Besacholine
6. Myotonachol
7. Carbamyl-beta-methylcholine Chloride
8. Mechotane
9. Mechothane
10. Mecothane
11. Mictone
12. Mictrol
13. Bethanechol (chloride)
14. Uro-carb
15. Urecholine Chloride
16. Carbamylmethylcholine Chloride
17. Bethaine Choline Chloride
18. 2-carbamoyloxypropyltrimethylammonium Chloride
19. Trimethyl(2-carbamoyloxypropyl)ammonium Chloride
20. 2-(carbamoyloxy)-n,n,n-trimethylpropan-1-aminium Chloride
21. (2-hydroxypropyl)trimethylammonium Chloride Carbamate
22. 2-(carbamoyloxy)-n,n,n-trimethyl-1-propanaminium Chloride
23. Beta-methyl Carbachol Chloride
24. Nsc-30783
25. 2-((aminocarbonyl)oxy)-n,n,n-trimethyl-1-propanaminium Chloride
26. 1-propanaminium, 2-[(aminocarbonyl)oxy]-n,n,n-trimethyl-, Chloride
27. H4qbz2lo84
28. Mls000028675
29. Chebi:3085
30. Myotonine
31. 2-carbamoyloxypropyl(trimethyl)azanium;chloride
32. Muscaran
33. Smr000058680
34. 1-propanaminium, 2-((aminocarbonyl)oxy)-n,n,n-trimethyl-, Chloride
35. Dsstox_cid_2676
36. Mfcd00055224
37. (2-carbamoyloxypropyl)trimethylammonium Chloride
38. Dsstox_rid_76686
39. Dsstox_gsid_22676
40. Myotonine Chloride
41. Beta-methylcholine Chloride Urethan
42. Sr-01000000282
43. Ncgc00163217-01
44. Einecs 209-686-8
45. Nsc 30783
46. Unii-h4qbz2lo84
47. Carbaminoyl, Beta-methylcholine Chloride
48. 1-propanaminium, 2-((aminocarbonyl)oxy)-n,n,n-trimethyl-, Chloride (1:1)
49. 1-propanaminium, 2-[(aminocarbonyl)oxy]-n,n,n-trimethyl-, Chloride (1:1)
50. Carbamyl-
51. A-methylcholine Chloride
52. Urecholine (tn)
53. Bethanechol Chloride [usp:ban:jan]
54. Regid855684
55. Opera_id_1125
56. Cas-590-63-6
57. Chembl1768
58. Schembl37393
59. Ammonium, Chloride, Carbamate
60. Mls001148071
61. Mls006011984
62. Spectrum1500146
63. Dtxsid2022676
64. .beta.-methyl Carbachol Chloride
65. Hms500i21
66. Hy-b0406a
67. (+-)-(2-hydroxypropyl)trimethylammonium Chloride Carbamate
68. Bethanechol Chloride [mi]
69. Carbamyl-ss-methylcholine Chloride
70. Bethanechol Chloride (jp17/usp)
71. Bethanechol Chloride [jan]
72. Hms1571g08
73. Hms1920g19
74. Hms2091m19
75. Hms2098g08
76. Hms2232d09
77. Hms3259l15
78. Hms3260n10
79. Hms3374d01
80. Hms3656g21
81. Hms3715g08
82. Hms3884h19
83. Pharmakon1600-01500146
84. Nsc30783
85. Bethanechol Chloride [vandf]
86. Tox21_112028
87. Tox21_500304
88. Ammonium, (2-hydroxypropyl)trimethyl-, Chloride, Carbamate
89. Bethanechol Chloride [mart.]
90. Ccg-38936
91. Nsc755913
92. S2455
93. .beta.-methylcholine Chloride Urethan
94. Bethanechol Chloride [usp-rs]
95. Bethanechol Chloride [who-dd]
96. 1-propanaminium, 2-((aminocarbonyl)oxy)-n,n,n-trimethyl-, Chloride, (+-)-
97. Akos006230324
98. Carbamyl- Beta -methylcholine Chloride
99. Tox21_112028_1
100. Carbamyl-beta-methylcholine Chloride.cd
101. Lp00304
102. Nc00660
103. Nsc-755913
104. Ncgc00015245-10
105. Ncgc00093753-01
106. Ncgc00093753-02
107. Ncgc00093753-03
108. Ncgc00093753-04
109. Ncgc00093753-05
110. Ncgc00260989-01
111. ( Inverted Exclamation Marka)-bethanechol
112. As-13371
113. Bethanechol Chloride [orange Book]
114. Carbaminoyl, .beta.-methylcholine Chloride
115. Wln: Zvoy1&1k1&1&1 &q &g
116. Bethanechol Chloride [usp Monograph]
117. Carbamyl-b-methylcholinechloride*crystalline
118. Eu-0100304
119. Ft-0622944
120. Ft-0663092
121. Ft-0663093
122. Sw197254-3
123. En300-51035
124. C 5259
125. C08202
126. D01000
127. F20466
128. A832139
129. Sr-01000000282-2
130. Sr-01000000282-4
131. Sr-01000000282-7
132. Q27105939
133. (+/-)-(2-hydroxypropyl)trimethylammonium Chloride Carbamate
134. 2-[(aminocarbonyl)oxy]-n,n,n-trimethylpropan-1-aminium Chloride
135. 1-propanaminium, 2-((aminocarbonyl)oxy)-n,n,n-trimethyl-, Chloride, (+/-)-
136. 2-carbamoyloxypropyl(trimethyl)ammonium Chloride;2-(carbamoyloxy)-n,n,n-trimethyl-1-propanaminium Chloride
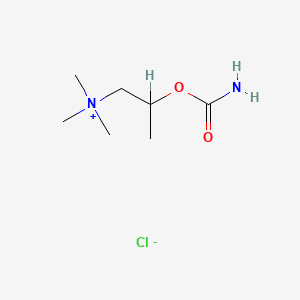
| Molecular Weight | 196.67 g/mol |
|---|---|
| Molecular Formula | C7H17ClN2O2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 196.0978555 g/mol |
| Monoisotopic Mass | 196.0978555 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Bethanechol chloride |
| Drug Label | Bethanechol chloride, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.It is designated chemically as 2-[(aminocarbonyl)oxy]-N, N, N-trimethyl-1-propanaminium chloride. Its molecular formu... |
| Active Ingredient | Bethanechol chloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 25mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Emcure Usa; Wockhardt; Upsher Smith; Amneal Pharm; Lannett; Sun Pharm Inds; Pharmax; Impax Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Urecholine |
| PubMed Health | Bethanechol |
| Drug Classes | Cholinergic, Urinary Antispasmodic |
| Active Ingredient | Bethanechol chloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 5mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Odyssey Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Bethanechol chloride |
| Drug Label | Bethanechol chloride, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.It is designated chemically as 2-[(aminocarbonyl)oxy]-N, N, N-trimethyl-1-propanaminium chloride. Its molecular formu... |
| Active Ingredient | Bethanechol chloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 25mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Emcure Usa; Wockhardt; Upsher Smith; Amneal Pharm; Lannett; Sun Pharm Inds; Pharmax; Impax Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Urecholine |
| PubMed Health | Bethanechol |
| Drug Classes | Cholinergic, Urinary Antispasmodic |
| Active Ingredient | Bethanechol chloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 5mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Odyssey Pharms |
Muscarinic Agonists
Drugs that bind to and activate muscarinic cholinergic receptors (RECEPTORS, MUSCARINIC). Muscarinic agonists are most commonly used when it is desirable to increase smooth muscle tone, especially in the GI tract, urinary bladder and the eye. They may also be used to reduce heart rate. (See all compounds classified as Muscarinic Agonists.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
