


1. 149877-41-8
2. Floramite
3. Bifenazate [iso]
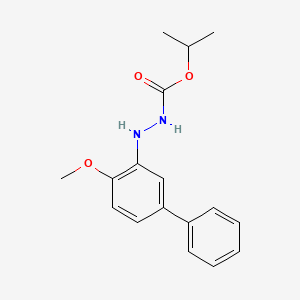
4. Isopropyl 2-(4-methoxy-[1,1'-biphenyl]-3-yl)hydrazinecarboxylate
5. Propan-2-yl N-(2-methoxy-5-phenylanilino)carbamate
6. Chebi:38660
7. Bifenazate 100 Microg/ml In Toluene
8. 24z9qw0505
9. Bifenazate 1000 Microg/ml In Toluene
10. Isopropyl 2-(4-methoxybiphenyl-3-yl)hydrazinecarboxylate
11. Bifenazate 100 Microg/ml In Acetonitrile
12. Isopropyl 2-(4-methoxy-[1,1'-biphenyl]-3-yl)hydrazine-1-carboxylate
13. Propan-2-yl 2-(4-methoxy[1,1'-biphenyl]-3-yl)hydrazinecarboxylate
14. 2-(4-methoxy(1,1'-biphenyl)-3-yl)hydrazinecarboxylic Acid 1-methylethyl Ester
15. Hsdb 7002
16. Epa Pesticide Chemical Code 000586
17. Ccris 9247
18. Unii-24z9qw0505
19. D 2341
20. Bifenazate [mi]
21. Bifenazate [hsdb]
22. Dsstox_cid_12525
23. Dsstox_rid_78968
24. Dsstox_gsid_32525
25. Schembl26414
26. Chembl1872845
27. Dtxsid5032525
28. Amy3610
29. Bcp06313
30. Zinc2511856
31. Tox21_300871
32. Mfcd03792817
33. Akos015900766
34. Hydrazinecarboxylic Acid, 2-(4-methoxy(1,1'-biphenyl)-3-yl)-, 1-methylethyl Ester
35. Ncgc00163782-01
36. Ncgc00163782-02
37. Ncgc00163782-03
38. Ncgc00254775-01
39. Db-063837
40. Hy-119687
41. Cas-149877-41-8
42. Cs-0077815
43. Ft-0736041
44. Bifenazate, Pestanal(r), Analytical Standard
45. C18589
46. D-2341
47. J-519850
48. Isopropyl 3-(4-methoxybiphenyl-3-yl)carbazate
49. Q13439316
50. Isopropyl 2-(4-methoxy-3-biphenylyl)hydrazinecarboxylate
51. Propan-2-yl N-[(2-methoxy-5-phenylphenyl)amino]carbamate
52. 1-methylethyl 2-(4-methoxybiphenyl-3-yl)hydrazinecarboxylate
53. 1-methylethyl 2-(4-methoxy(1,1'-biphenyl)-3-yl)hydrazinecarboxylate
54. Pesticide3_bifenazate_c17h20n2o3_1-methylethyl 2-(4-methoxybiphenyl-3-yl)hydrazinecarboxylate
| Molecular Weight | 300.35 g/mol |
|---|---|
| Molecular Formula | C17H20N2O3 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 300.14739250 g/mol |
| Monoisotopic Mass | 300.14739250 g/mol |
| Topological Polar Surface Area | 59.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 343 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sprague-Dawley Crl:CD/BR rats of both sexes were dosed with either 10 or 1,000 mg/kg of [(14)C] ... /bifenazate/ ... (radiochemical purity: >98%, specific activity: 34.4 mCi/mmol, label on the aromatic ring, /bifenazate/... (unlabeled ... purity: 99.6%) by oral gavage. In the Distribution, Metabolism and Excretion study, 5 animals/sex/group were dosed and urine and feces were collected for 7 days. In the Biliary study, 3 animals/sex/group with cannulated bile ducts were dosed and urine, feces and bile were collected for 72 hrs. The Pilot Pharmacokinetic and Pharmacokinetic studies were performed in which 3 animals/sex/group and 5 animals/sex/group, respectively, were dosed. In the pilot study, urine and feces were collected for 4 days and blood samples were collected via a jugular cannula for 72 hrs. In the main study, urine and blood were collected for 4 days from the animals in the 10 mg/kg treatment group. In the 1,000 mg/kg group, urine, feces and blood were collected for 7 days. In the Tissue Distribution study, 9 animals/sex/group were dosed. In the 10 mg/kg group, 3 animals/sex/time point were serially euthanized at 6, 24 and 48 hrs after dosing. In the 1,000 mg/kg group, 3 animals/sex/time point were euthanized at 18, 42 and 72 hrs post-dose. Urine and feces were collected at designated intervals. Tissue samples from Distribution, Metabolism and Excretion study and the Tissue Distribution study were processed for the presence of radiolabel. Radiolabeled materials were isolated from the urine and feces derived from the Distribution, Metabolism and Excretion and the Biliary studies and structurally analyzed for a metabolic profile. The predominant route of excretion for both doses was via the feces. For the 10 mg/kg group, 66% of the administered dose (AD) was recovered in the feces with 75-82% of that total excreted in the first 24 hrs. Radiolabel recovered in the urine and cage wash constituted 27-29% of the AD after 7 days. For the 1,000 mg/kg treatment group, the % of AD recovered in the feces up to 7 days post-dose was 82% with 46-57% of that total recovered in the first 24 hrs. The % of AD isolated in the urine and cage wash was 10-15%. The Biliary study demonstrated that the bile was a significant pathway for excretion by the 10 mg/kg treatment group with 69-74% of the administered dose recovered in the bile up to 72 hrs after dosing. In contrast, for the high dose group, 21-26% of the dose was isolated in the bile by 72 hrs. Recovery in the feces of the 10 mg/kg group was limited to 7-8% of the AD as compared to 56-64% of the AD for the 1,000 mg/kg group. A significant fraction of the AD for the high dose group was not being absorbed. The following pharmacokinetic parameters were derived: tmax, 5 and 6 hrs and 18- 24 hrs for males and females of the 10 mg/kg and 1,000 mg/kg groups, respectively, Cmax, 6.4 and 5.6 ug equiv./g and 119 and 71 ug equiv./g for the males and females in the 10 mg/kg and 1,000 mg/kg groups, respectively, and t1/2, 11.5 and 13.3 hrs and 12 and 15.6 hrs for males and females in the low and high dose groups, respectively. In the Tissue Distribution study, among the 10 mg/kg animals, maximal residue levels were noted at 6 hrs with none of the radiolabel being sequestered in any of the tissues. In the 1,000 mg/kg group, maximal residue levels were noted in a majority of the tissues at 18 hrs post-dose for the males and 42 hrs post-dose for the females. For some of the organs, appreciable levels of radiolabel were still evident at 7 days (e.g., spleen, red blood cell, liver, and kidney). Analysis of the radiolabeled moieties recovered in the feces revealed a number of modifications of the parent cmpd. Hydrazine oxidation, demethylation, ring hydroxylation, separation into biphenyl and hydrazinecarboxylic acid moieties and conjugation with glucuronic acid or sulfate. For the 10 mg/kg group, identified moieties extracted from the feces constituted 39% of the AD. The predominant compounds were ... /bifenazate/ glucuronide (6.3-8.9% of AD), ... /bifenazate/ (4.8-7.2% of AD) and ... biphenyl, 4-hydroxy (5.5-7.1% of AD). In contrast, for the 1,000 mg/kg group, the parent cmpd which was recovered in the feces constituted 48-61% of the AD. /Bifenazate/... glucuronide constituted 4.7-5.6% of the AD ... Overall, the test material was well absorbed at the low dose, metabolized and conjugated before being excreted in the bile. At the high dose level, a much lower % of the dose was absorbed.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Bifenazate, Chemical Code No.5657 p.7-8 (March 30, 2000, last revised August 25, 2000). Available from, as of December 19, 2016: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Sprague-Dawley Crl:CD/BR rats of both sexes were dosed with either 10 or 1,000 mg/kg of [(14)C] ... /bifenazate/ ( ... radiochemical purity: >98%, specific activity: 34.4 mCi/mmol, label on the aromatic ring, ... /bifenazate/ (unlabeled ... purity: 99.6%) by oral gavage. ... Analysis of the radiolabeled moieties recovered in the feces revealed a number of modifications of the parent cmpd. Hydrazine oxidation, demethylation, ring hydroxylation, separation into biphenyl and hydrazinecarboxylic acid moieties and conjugation with glucuronic acid or sulfate. For the 10 mg/kg group, identified moieties extracted from the feces constituted 39% of the administered dose (AD). The predominant compounds were ... /bifenazate/ glucuronide (6.3-8.9% of AD), ... /bifenazate/ (4.8-7.2% of AD) and ... biphenyl, 4-hydroxy (5.5-7.1% of AD). In contrast, for the 1,000 mg/kg group, the parent cmpd which was recovered in the feces constituted 48-61% of the AD. ... /Bifenazate/ glucuronide constituted 4.7-5.6% of the AD. The primary moieties recovered in the urine were conjugates of ... p, p-biphenol or sulfates of ... /p, p'-biphenol/ and ... /biohenyl, 4-hydroxy/. The total of these cmpds constituted 19-21% of the administered dose for the 10 mg/kg group and 6-7% of the administered dose for the 1,000 mg/kg group. Major metabolites identified in the bile were ... /biphenyl, 4-hydroxy/ (17-20% of AD in the 10 mg/kg group and 2.1-2.5% of the AD in the 1,000 mg/kg group), ... biphenyl, 4-hydroxy, 4-methoxy (17-19% of the AD in the 10 mg/kg and 2.8% of the AD in the 1,000 mg/kg group) and ... /bifenazate/ glucuronide (9-12% of the AD in the 10 mg/kg and 9-13% of the AD in the 1,000 mg/kg group).
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Bifenazate, Chemical Code No.5657 p.7-8 (March 30, 2000, last revised August 25, 2000). Available from, as of December 19, 2016: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Sprague-Dawley Crl:CD/BR rats of both sexes were dosed with either 10 or 1,000 mg/kg of [(14)C] ... /bifenazate/ ... (radiochemical purity: >98%, specific activity: 34.4 mCi/mmol, label on the aromatic ring, /bifenazate/ ... (unlabeled ... purity: 99.6%) by oral gavage. ... The following pharmacokinetic parameters were derived: tmax, 5 and 6 hrs and 18-24 hrs for males and females of the 10 mg/kg and 1,000 mg/kg groups, respectively, Cmax, 6.4 and 5.6 ug equiv./g and 119 and 71 ug equiv./g for the males and females in the 10 mg/kg and 1,000 mg/kg groups, respectively, and t1/2, 11.5 and 13.3 hrs and 12 and 15.6 hrs for males and females in the low and high dose groups, respectively.
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data, Bifenazate, Chemical Code No.5657 p.7 (March 30, 2000, last revised August 25, 2000). Available from, as of December 19, 2016: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm