


1. 202189-78-4
2. Bilaxten
3. Ilaxten
4. Bilastine [inn]
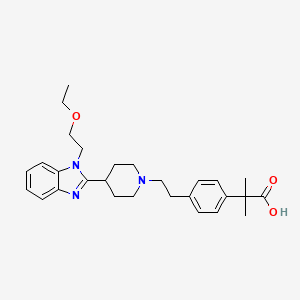
5. 2-[4-[2-[4-[1-(2-ethoxyethyl)benzimidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylpropanoic Acid
6. 2-(4-(2-(4-(1-(2-ethoxyethyl)-1h-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic Acid
7. Pa1123n395
8. Bilastinum
9. Bilatex
10. Unii-pa1123n395
11. 2-[4-[2-[4-[1-(2-ethoxyethyl)-1h-benzo[d]imidazol-2-yl]piperidin-1-yl]ethyl]phenyl]-2-methylpropanoic Acid
12. Bilanoa (tn)
13. Bilastine (jan/inn)
14. Bilastine [jan]
15. Bilastine [mi]
16. Bilastine [who-dd]
17. Dsstox_cid_31467
18. Dsstox_rid_97352
19. Dsstox_gsid_57678
20. Benzeneacetic Acid,4-[2-[4-[1-(2-ethoxyethyl)-1h-benzimidazol-2-yl]-1-piperidinyl]ethyl]-a,a-dimethyl-
21. Schembl991810
22. Chembl1742423
23. Dtxsid5057678
24. Gtpl11579
25. Chebi:135954
26. Hms3887o17
27. Amy16470
28. Bcp02576
29. Ex-a2962
30. Zinc3822702
31. Tox21_113905
32. Mfcd09837814
33. S3721
34. Akos030241723
35. Bcp9000412
36. Ccg-269384
37. Db11591
38. F-96221-bm
39. Sb17508
40. Ncgc00262907-01
41. Ac-29231
42. Bs-15792
43. Hy-14447
44. B5392
45. Cas-202189-78-4
46. Ft-0700542
47. D09570
48. 189b784
49. A856214
50. Q2902977
51. P-(2-(4-(1-(2-ethoxyethyl)-2-benzimidazolyl)piperidino)ethyl)-alpha-methylhydratropic Acid
52. 2-[4-(2-{4-[1-(2-ethoxy-ethyl)-1h-benzoimidazol-2-yl]-piperidin-1-yl}-ethyl)-phenyl]-2-methyl-propionic Acid
53. 2-[4-(2-{4-[1-(2-ethoxy-ethyl)-1h-benzoimidazol-2-yl]-piperidin-1-yl}ethyl)-phenyl]-2-methyl-propionic Acid
54. 2-[4-[2-[4-[1-(2-ethoxyethyl)-1h-benzoimidazole-2-yl]piperidine-1-yl]ethyl]phenyl]-2-methylpropanoic Acid
55. 2-[4-[2-[4-[1-(2-ethoxyethyl)benzoimidazol-2-yl]-1-piperidyl]ethyl]phenyl]-2-methyl-propanoic Acid
56. Benzeneacetic Acid, 4-(2-(4-(1-(2-ethoxyethyl)-1h-benzimidazol-2-yl)-1-piperidinyl)ethyl-alpha, Alpha-dimethyl-
57. Benzeneacetic Acid, 4-[2-[4-[1-(2-ethoxyethyl)-1h-benzimidazol-2-yl]-1-piperidinyl]ethyl]-alpha,alpha-dimethyl-
58. Benzeneaceticacid,4-(2-(4-(1-(2-ethoxyethyl)-1h-benzimidazol-2-yl)-1-piperidinyl)ethyl-alpha,alpha-dimethyl-
59. Bilastine; 2-(4-(2-(4-(1-(2-ethoxyethyl)-1h-benzo[d]imidazol-2-yl)piperidin-1-yl)ethyl)phenyl)-2-methylpropanoic Acid
60. P-(2-(4-(1-(2-ethoxyethyl)-2-benzimidazolyl)piperidino)ethyl)-.alpha.-methylhydratropic Acid
| Molecular Weight | 463.6 g/mol |
|---|---|
| Molecular Formula | C28H37N3O3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 463.28349205 g/mol |
| Monoisotopic Mass | 463.28349205 g/mol |
| Topological Polar Surface Area | 67.6 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 641 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For symptomatic relief of nasal and non-nasal symptoms of seasonal rhinitis in patients 12 years of age and older and for symptomatic relief in chronic spontaneous urticaria in patients 18 years of age and older.
FDA Label
Treatment of allergic rhinoconjunctivitis, Treatment of urticaria
Treatment of urticaria, Treatment of allergic rhinoconjunctivitis
Treatment of acute type I hypersensitivity reactions
Treatment of allergic conjunctivitis
Bilastine is an antiallergenic and acts to reduce allergic symptoms such as nasal congestion and urticaria.
R06AX29
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AX - Other antihistamines for systemic use
R06AX29 - Bilastine
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX13 - Bilastine
Absorption
Bilastine has a Tmax of 1.13 h. The absolute bioavailability is 61%. No accumulation observed with daily dosing of 20-100 mg after 14 days. Cmax decreased by 25 % and 33% when taken with a low fat and high fat meal compared to fasted state. Administration with grapefruit juice decreased Cmax by 30%.
Route of Elimination
Bilastine is mainly excreted in the feces (66.5%) with some excreted in the urine (28.3%). Nearly all is excreted as the parent compound.
Clearance
Bilastine has a total clearance is 9.20 L/h and a renal clearance of 8.7 L/h.
Bilastine does not interact with the cytochrome P450 system and does not undergo significant metabolism in humans.
The mean half life of elimination is 14.5h.
Bilastine is a selective histamine H1 receptor antagonist (Ki = 64nM). During allergic response mast cells undergo degranulation which releases histamine and other subastances. By binding to and preventing activation of the H1 receptor, bilastine reduces the development of allergic symptoms due to the release of histamine from mast cells.
