

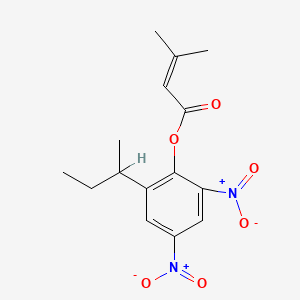

1. Binapacrile
2. Binapakril
1. 485-31-4
2. Endosan
3. Dinapacryl
4. Acricid
5. Dapacryl
6. Morocide
7. Morrocid
8. Ambox
9. Dinoseb Methacrylate
10. Niagara 9044
11. Hoe 2784 Oa
12. Dinoseb, 3,3-dimethylacryl Ester
13. 2-(1-methylpropyl)-4,6-dinitrophenyl 3-methyl-2-butenoate
14. Fmc 9044
15. Hoe 2784
16. Nia 9044
17. Ent 25,793
18. 2-sec-butyl-4,6-dinitrophenyl Senecioate
19. (2-butan-2-yl-4,6-dinitrophenyl) 3-methylbut-2-enoate
20. 2,4-dinitro-6-sec-butylphenyl 2-methylcrotonate
21. 2-sec-butyl-4,6-dinitrophenyl 3-methylcrotonate
22. 2-sec-butyl-4,6-dinitrophenyl 3-methyl-2-butenoate
23. 2-sec-butyl-4,6-dinitrophenyl-3,3-dimethylacrylate
24. 4,6-dinitrophenyl-2-sec-butyl-3-methyl-2-butenonate
25. 2-butenoic Acid, 3-methyl-, 2-(1-methylpropyl)-4,6-dinitrophenyl Ester
26. Phenol, 2-sec-butyl-4,6-dinitro-, 3-methylcrotonate
27. 3-methylcrotonic Acid 2-sec-butyl-4,6-dinitrophenyl Ester
28. 3,3-dimethylacrylic Acid 2-sec-butyl-4,6-dinitrophenyl Ester
29. 3-methyl-2-butenoic Acid 2-sec-butyl-4,6-dinitrophenyl Ester
30. Chebi:83366
31. Crotonic Acid, 3-methyl-, 2-sec-butyl-4,6-dinitrophenyl Ester
32. 3-methyl-2-butenoic Acid 2-(1-methylpropyl)-4,6-dinitrophenyl Ester
33. 4x685bb13a
34. 2-sek.butyl-4,6-dinitrofenylester Kyseliny 3-methylkrotonove
35. (6-(1-metil-propil)-2,4-dinitro-fenil)-3,3-dimetil-acrilato
36. (6-(1-methyl-propyl)-2,4-dinitro-fenyl)-3,3-dimethyl-acrylaat
37. (6-(1-methyl-propyl)-2,4-dinitro-phenyl)-3,3-dimethyl-acrylat
38. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle) Phenyle
39. 2-sec-butyl-4,6-dinitrophenyl 3-methylbut-2-enoate
40. Caswell No. 086
41. 4,6-dinitrophenyl-2-(1-methylpropyl) 3-methylbut-2-enoate
42. 2-butenoic Acid,3-methyl-, 2-(1-methylpropyl)-4,6-dinitrophenyl Ester
43. 2-butenoic Acid, 3-methyl-, 2-(1-methylpropyl)-4,6-dinitrophenyl Ester (9ci)
44. Binapacryl [ansi:bsi:iso]
45. Hsdb 1518
46. Einecs 207-612-9
47. Epa Pesticide Chemical Code 012201
48. Brn 2013712
49. Unii-4x685bb13a
50. Ai3-25793
51. Senecioic Acid 2-sec Butyl-4,6-dinitrophenyl Ester
52. Senecioic Acid 2-sec-butyl-4,6-dinitrophenyl Ester
53. 2-sec-butyl-4,6-dinitrophenyl Beta,beta-dimethylacrylate
54. 4,6-dinitro-2-sec-butylphenyl Beta,beta-dimethylacrylate
55. 2-(1-methylpropyl)-4,6-dinitrophenyl 3,3-dimethylacrylate
56. Binapacryl [mi]
57. 2-(1-methylpropyl)-4,6-dinitrophenyl Beta,beta-dimethacrylate
58. Binapacryl [iso]
59. Binapacryl [hsdb]
60. (6-(1-methyl-propyl)-2,4-dinitro-fenyl)-3,3-dimethyl-acrylaat [dutch]
61. (6-(1-metil-propil)-2,4-dinitro-fenil)-3,3-dimetil-acrilato [italian]
62. 2-sek.butyl-4,6-dinitrofenylester Kyseliny 3-methylkrotonove [czech]
63. (6-(1-methyl-propyl)-2,4-dinitro-phenyl)-3,3-dimethyl-acrylat [german]
64. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle) Phenyle [french]
65. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle)phenyl [french]
66. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle)phenyle [french]
67. Dsstox_cid_20269
68. Dsstox_rid_79462
69. Dsstox_gsid_40269
70. Schembl54301
71. Chembl3182974
72. Dtxsid9040269
73. Zrdusmywdrpzrm-uhfffaoysa-
74. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle)phenyl
75. 3,3 Dimethyl-acrylate De 2,4-dinitro-6-(1-methylpropyle)phenyle
76. Tox21_301554
77. Mfcd00055425
78. Akos015888368
79. Binapacryl 1000 Microg/ml In Hexane
80. Binapacryl 10 Microg/ml In Cyclohexane
81. Ncgc00255512-01
82. Cas-485-31-4
83. Db-051558
84. Ft-0603417
85. Binapacryl, Pestanal(r), Analytical Standard
86. C19022
87. Q3468507
88. (rs)-2-sec-butyl-4,6-dinitrophenyl 3-methylcrotonate
89. 2-(butan-2-yl)-4,6-dinitrophenyl 3-methylbut-2-enoate
90. 2-sec-butyl-4,6-dinitrophenyl 3-methylcrotonate (binapacryl)
91. 4,6-dinitro-2-sec-butylphenyl .beta.,.beta.-dimethylacrylate
92. 2-(1-methylpropyl)-4,6-dinitrophenyl .beta.,.beta.-dimethacrylate
| Molecular Weight | 322.31 g/mol |
|---|---|
| Molecular Formula | C15H18N2O6 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 322.11648630 g/mol |
| Monoisotopic Mass | 322.11648630 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 489 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
BINAPACRYL WAS ABSORBED POORLY, IF AT ALL, THROUGH SKIN OF MICE AND RABBITS.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 170
After a single administration of binapacryl, both rats and rabbits excreted 7-17% in the urine within 48 hr. As late as the tenth day, 0.12% of the dose was detected in the urine of rats ... .
Hayes, Wayland J., Jr. Pesticides Studied in Man. Baltimore/London: Williams and Wilkins, 1982., p. 470
WHEN ADMINISTERED ORALLY TO GUINEA PIGS, THIS COMPOUND GIVES RISE TO ... /2-SEC-BUTYL-4,6-DINITROPHENOL/ IN BLOOD.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 170
Following oral doses of binapacryl, the urine of both rabbits and rats contained oxidation products of the butyl side chain of dinoseb, including the acid. In addition, rat urine contained traces of free 6-aminophenol and the urine of rabbits contained a substantial amount of this compound and its glucuronide.
Hayes, Wayland J., Jr. Pesticides Studied in Man. Baltimore/London: Williams and Wilkins, 1982., p. 470
BASIC MECHANISM OF TOXICITY IS STIMULATION OF OXIDATIVE METABOLISM IN CELL MITOCHONDRIA, BY INTERFERENCE WITH NORMAL COUPLING OF CARBOHYDRATE OXIDN TO PHOSPHORYLATION (ADP TO ATP). INCR OXIDATIVE METABOLISM LEADS TO PYREXIA, TACHYCARDIA, & DEHYDRATION, & ULTIMATELY DEPLETES CARBOHYDRATE & FAT STORES. /NITROPHENOLIC COMPOUNDS/
Morgan, D.P. Recognition and Management of Pesticide Poisonings. EPA 540/9-80-005. Washington, DC: U.S. Government Printing Office, Jan. 1982., p. 23