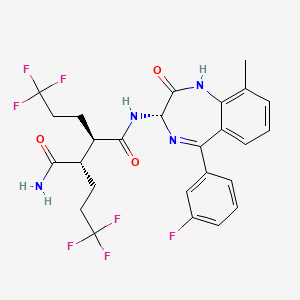

1. (2r,3s)-n-((3s)-5-(3-fluorophenyl)-9-methyl-2-oxo-2,3-dihydro-1h-1,4-benzodiazepin-3-yl)-2,3-bis(3,3,3-trifluoropropyl)succinamide
2. Bms-986115
3. Butanediamide, N1-((3s)-5-(3-fluorophenyl)-2,3-dihydro-9-methyl-2-oxo-1h-1,4-benzodiazepin-3-yl)-2,3-bis(3,3,3-trifluoropropyl)-, (2r,3s)-
1. Bms-986115
2. 1584647-27-7
3. Notch Inhibitor 1
4. Bms 986115
5. Lsk1l593uu
6. (2s,3r)-n'-[(3s)-5-(3-fluorophenyl)-9-methyl-2-oxo-1,3-dihydro-1,4-benzodiazepin-3-yl]-2,3-bis(3,3,3-trifluoropropyl)butanediamide
7. Chembl3911164
8. Schembl15608290
9. Bms986115
10. Nsc836050
11. Bms 986115 [who-dd]
12. Db13126
13. Nsc-836050
14. (2r,3s)-n-((3s)-5-(3-fluorophenyl)-9-methyl-2-oxo-2,3-dihydro-1h-1,4-benzodiazepin-3-yl)-2,3-bis(3,3,3-trifluoropropyl)succinamide
15. Hy-12860
16. Cs-0012731
17. P14960
18. Us9273014, 1
19. Q27283163
20. (2r,3s)-n1-((s)-5-(3-fluorophenyl)-9-methyl-2-oxo-2,3-dihydro-1h-benzo[e][1,4]diazepin-3-yl)-2,3-bis(3,3,3-trifluoropropyl)succinamide
21. Butanediamide, N1-((3s)-5-(3-fluorophenyl)-2,3-dihydro-9-methyl-2-oxo-1h-1,4-benzodiazepin-3-yl)-2,3-bis(3,3,3-trifluoropropyl)-, (2r,3s)-
| Molecular Weight | 574.5 g/mol |
|---|---|
| Molecular Formula | C26H25F7N4O3 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 574.18148781 g/mol |
| Monoisotopic Mass | 574.18148781 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 956 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Gamma Secretase Inhibitors and Modulators
Agents that suppress GAMMA-SECRETASE by inhibiting or modulating its activities. Targeted enzymatic activities include its involvement in accumulation of toxic AMYLOID BETA-PEPTIDES (e.g., Aβ42) in ALZHEIMER DISEASE and activation of NOTCH RECEPTOR mediated SIGNAL PATHWAYS in certain cancer types. (See all compounds classified as Gamma Secretase Inhibitors and Modulators.)