


1. Ski-606
2. Ski606
1. 380843-75-4
2. Ski-606
3. Bosutinib (ski-606)
4. Ski 606
5. Bosulif
6. Ski606
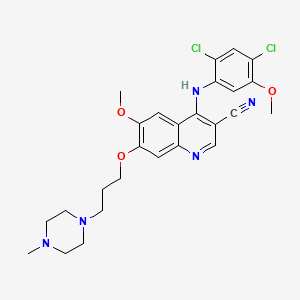
7. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile
8. 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3-carbonitrile
9. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile
10. Bosutinib (usan)
11. Bosutinib [usan]
12. Sk-606
13. 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methyl-1-piperazinyl)propoxy)-3-quinolinecarbonitrile
14. Bosutinib Isomer 1
15. Chembl288441
16. 4-(2,4-dichloro-5-methoxyanilino)-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile
17. 4-(2,4-dichloro-5-methoxyphenylamino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3-carbonitrile
18. Chebi:39112
19. 5018v4aez0
20. Ski-606)
21. Bosutinib (as Monohydrate)
22. Bosutinib [usan:inn]
23. Ncgc00241107-01
24. Unii-5018v4aez0
25. Mfcd07367846
26. Bosutinib,ski-606
27. Bosutinib [inn]
28. Bosutinib (ski606)
29. Bosutinib [mi]
30. Bosutinib [vandf]
31. Pf-5208763
32. Bosutinib - Ski-606
33. Bosutinib [mart.]
34. Ec 700-455-1
35. Bosutinib [who-dd]
36. Mls006011212
37. Schembl158390
38. Amy266
39. Bdbm4552
40. Gtpl5710
41. Bosutinib, >=98% (hplc)
42. Dtxsid10861568
43. Ex-a391
44. Bcpp000318
45. Hms2043a22
46. Hms3244a03
47. Hms3244a04
48. Hms3244b03
49. Hms3651c03
50. Hms3672k11
51. Hms3743e09
52. 2-pyridin-2-ylethylacetate
53. K00615a
54. Bcp01782
55. Nsc765694
56. Nsc799367
57. Sk 606
58. Zinc22448983
59. Akos015902521
60. Ac-2413
61. Bcp9000446
62. Ccg-208129
63. Cs-0118
64. Db06616
65. Nsc-765694
66. Nsc-799367
67. Pb30881
68. Ncgc00241107-03
69. Ncgc00241107-05
70. 4-(2,4-dichloro-5-methoxy-anilino)-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile
71. As-11064
72. Hy-10158
73. Smr002530350
74. Ft-0656231
75. S1014
76. Ski606; Ski 606; Sk-i606
77. Sw220197-1
78. A25014
79. D03252
80. Ab01565836_03
81. 843b754
82. Q894611
83. Sr-01000941572
84. J-519931
85. Q-200745
86. Sr-01000941572-1
87. Brd-k99964838-001-01-0
88. Brd-k99964838-001-06-9
89. 3-quinolinecarbonitrile, 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methyl-1-piperazinyl)propoxy)-
90. 3-quinolinecarbonitrile, 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methyl-1-piperazinyl)propoxy)-
91. 4-(2,4-dichloro-5-methoxy-phenylamino)-6-methoxy-7-[3-(4-methyl-piperazin-1-yl)propoxy]quinoline-3-carbonitrile
92. Ski-606;4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile
| Molecular Weight | 530.4 g/mol |
|---|---|
| Molecular Formula | C26H29Cl2N5O3 |
| XLogP3 | 5.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 529.1647452 g/mol |
| Monoisotopic Mass | 529.1647452 g/mol |
| Topological Polar Surface Area | 82.9 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 734 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | <a class="pubchem-internal-link multiple-CIDs" href="/compound/Bosulif">Bo |
| PubMed Health | <a class="pubchem-internal-link CID-5328940" href="/compound/Bosutinib" |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Bosutinib monohydrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 500mg base |
| Market Status | Prescription |
| Company | Wyeth Pharms |
Treatment of chronic, accelerated, or blast phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance or intolerance to prior therapy in adult patients.
FDA Label
Bosulif is indicated for the treatment of adult patients with:
- newlydiagnosed chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukaemia (Ph+ CML).
- CP, accelerated phase (AP), and blast phase (BP) Ph+ CML previously treated with one or more tyrosine kinase inhibitor(s) [TKI(s)] and for whom imatinib, nilotinib and dasatinib are not considered appropriate treatment options.
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EA - Bcr-abl tyrosine kinase inhibitors
L01EA04 - Bosutinib
Absorption
Food increase the exposure of bosutinib. Tmax, single dose, cancer patients, fed-state = 4-6 hours; After 15 daily doses of bosutinib 500 mg with food in CML patients, the pharmacokinetic parameters are as follows: Cmax = 200 ng/mL; AUC = 3650 ngh/mL
Route of Elimination
When given a single oral dose, 91.3% of the dose was recovered in feces and 3% of the dose recovered in urine.
Volume of Distribution
Apparent volume of distribution = 6080 1230 L.
Clearance
Mean clearance (CL/F), single oral dose, fed-state = 189 L/h
Bosutinib is primarily metabolized by CYP3A4. The major circulating metabolites identified in plasma are oxydechlorinated (M2) bosutinib (19% of parent exposure) and N-desmethylated (M5) bosutinib (25% of parent exposure), with bosutinib N-oxide (M6) as a minor circulating metabolite. All the metabolites were deemed inactive.
Terminal phase elimination half-life, single oral dose, fed-state = 22.5 hours
Bosutinib is a tyrosine kinase inhibitor. Although it is able to inhibit several tyrosine kinases such as Src, Lyn, and Hck, which are members of the Src-family of kinases, its primary target is the Bcr-Abl kinase. The Bcr-Abl gene is a chimeric oncogene created from the fusion of the breakpoint-cluster (Bcr) gene and Abelson (Abl) tyrosine gene. This chromosomal abnormality results in the formation of what is commonly known as the Philadelphia chromosome or Philadelphia translocation. The Bcr-Abl gene expresses a particular kinase that promotes the progression of CML. A decrease in the growth and size of the CML tumour has been observed following administration of bosutinib. Bosutinib did not inhibit the T315I and V299L mutant cells.