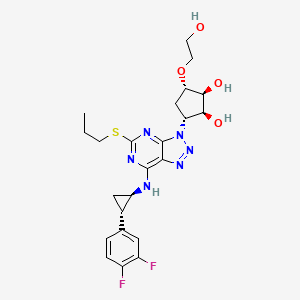

1. 3-(7-((2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3h-(1-3)-triazolo(4,5-d)pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
2. Azd 6140
3. Azd-6140
4. Azd6140
5. Brilinta
6. Brilique
1. 274693-27-5
2. Brilinta
3. Brilique
4. Azd6140
5. Azd-6140
6. Possia
7. Ar-c126532xx
8. Azd 6140
9. Ticargrelor
10. [14c]-ticagrelor
11. Glh0314rvc
12. (1s,2s,3r,5s)-3-(7-(((1r,2s)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
13. Chebi:68558
14. (1s,2s,3r,5s)-3-(7-((1r,2s)-2-(3,4-difluorophenyl)cyclopropylamino)-5-(propylthio)-3h-(1,2,3)triazolo(4,5-d)pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
15. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3h-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-1,2-cyclopentanediol
16. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
17. 1,2-cyclopentanediol, 3-(7-(((1r,2s)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3h-1,2,3-triazolo(4,5-d)pyrimidin-3-yl)-5-(2-hydroxyethoxy)-, (1s,2s,3r,5s)-
18. (1s,2s,3r,5s)-3-(7-((1r,2s)-2-(3,4-difluorophenyl)cyclopropylamino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
19. (1s,2s,3r,5s)-3-[7-[(1r,2s)-2-(3,4-difluorophenyl)cyclopropylamino]-5-(propylthio)- 3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
20. Ticagrelor [usan]
21. Unii-glh0314rvc
22. Ticagrelor [usan:inn:ban]
23. C23h28f2n6o4s
24. Ar-c 126532xx
25. Brilinta (tn)
26. (1s,2s,3r,5s)-3-(7-(((1r)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
27. (1s,2s,3r,5s)-3-(7-{[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylsulfanyl)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
28. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
29. (1s,2s,3r,5s)-3-[7-{[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylsulfanyl)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
30. Ticagrelor- Bio-x
31. Ar-c126532
32. Ticagrelor [mi]
33. Ticagrelor [inn]
34. Ticagrelor [jan]
35. Ticagrelor [vandf]
36. Ticagrelor [mart.]
37. Ticagrelor [usp-rs]
38. Ticagrelor [who-dd]
39. Ticagrelor (jan/usan/inn)
40. Ticagrelor [ema Epar]
41. Chembl398435
42. Gtpl1765
43. Schembl1979652
44. Ticagrelor [orange Book]
45. Ammd00027
46. Hsdb 8306
47. Ex-a503
48. Ticagrelor (brilinta,azd6140)
49. Ticagrelor [ep Monograph]
50. Dtxsid901009337
51. Hms3885b03
52. Bdbm50397205
53. Mfcd09954148
54. S4079
55. Zinc28957444
56. Akos015900739
57. Am85693
58. Ccg-269870
59. Cs-0619
60. Db08816
61. Ex-6274
62. Ar-c 126532xx;azd6140
63. Compound 17 [pmid: 17827008]
64. Ncgc00379052-01
65. Ncgc00379052-02
66. Ac-24755
67. As-19943
68. Bt164470
69. Hy-10064
70. D09017
71. Ab01565855_02
72. 693t275
73. A850981
74. Q420542
75. J-016772
76. J-524969
77. (1s,2s,3r,5s)-3-(7-(((1s,2s)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
78. (1s,2s,3r,5s)-3-(7-((1r,2s)-2-(3,4-difluorophenyl)cyclopropylamino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy) Cyclopentane-1,2-diol
79. (1s,2s,3r,5s)-3-(7-((1r,2s)-2-(3,4-difluorophenyl)cyclopropylamino)-5-(propylthio)-3h-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2 Diol
80. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-d1,2-cyclopentanediolifluorophenyl)cyclopropyl]amino]-5-(propylthio)-3h-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-1,2-cyclopentanediol
81. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)-cyclopropyl]amino]-5-(propylthio)-3h-[1,2,3]-triazolo[4,5-d]pyrimidine-3-yl]-5-(2-hydroxyethoxy) Cyclopentane-1,2-diol
82. (1s,2s,3r,5s)-3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[5,4-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
83. 1,2-cyclopentanediol,3-[7-[[(1r,2s)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3h-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-,(1s,2s,3r,5s)-
| Molecular Weight | 522.6 g/mol |
|---|---|
| Molecular Formula | C23H28F2N6O4S |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 522.18608089 g/mol |
| Monoisotopic Mass | 522.18608089 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 736 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Brilinta |
| PubMed Health | Ticagrelor (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | BRILINTA contains ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP-receptor. Chemically it is (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-... |
| Active Ingredient | Ticagrelor |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 90mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 2 of 2 | |
|---|---|
| Drug Name | Brilinta |
| PubMed Health | Ticagrelor (By mouth) |
| Drug Classes | Platelet Aggregation Inhibitor |
| Drug Label | BRILINTA contains ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP-receptor. Chemically it is (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-... |
| Active Ingredient | Ticagrelor |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 90mg |
| Market Status | Prescription |
| Company | Astrazeneca |
Purinergic P2Y Receptor Antagonists
National Library of Medicine's Medical Subject Headings. Ticagrelor. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ticagrelor is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=ticagrelor&Search=Search
Brilinta is indicated to reduce the rate of cardiovascular death, myocardial infarction, and stroke in patients with acute coronary syndrome (ACS) or a history of myocardial infarction (MI). For at least the first 12 months following ACS, it is superior to clopidogrel. /Included in US product label/
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
Brilinta also reduces the rate of stent thrombosis in patients who have been stented for treatment of acute coronary syndrome (ACS). /Included in US product label/
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
/BOXED WARNING/ BLEEDING RISK. Brilinta, like other antiplatelet agents, can cause significant, sometimes fatal bleeding. Do not use Brilinta in patients with active pathological bleeding or a history of intracranial hemorrhage. Do not start Brilinta in patients undergoing urgent coronary artery bypass graft surgery (CABG). If possible, manage bleeding without discontinuing Brilinta. Stopping Brilinta increases the risk of subsequent cardiovascular events
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 27, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
/BOXED WARNING/ ASPIRIN DOSE AND BRILINTA EFFECTIVENESS. Maintenance doses of aspirin above 100 mg reduce the effectiveness of Brilinta and should be avoided.
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 27, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
In general, treatment with ticagrelor should not be discontinued prematurely because this increases the risk of cardiovascular events. Premature discontinuance of antiplatelet therapy (e.g., P2Y12 adenosine diphosphate (ADP)-receptor antagonists, aspirin) in patients with coronary artery stents has been associated with an increased risk of ischemic cardiovascular events (e.g., stent thrombosis, myocardial infarction (MI), death). If temporary discontinuance of ticagrelor is necessary such as prior to elective surgery or for management of bleeding, the drug should be restarted as soon as possible. Patients should be advised to never stop taking ticagrelor without first consulting the prescribing clinician, even if instructed by another clinician (e.g., dentist) to stop such therapy. Prior to scheduling an invasive procedure, patients should inform clinicians (including dentists) that they are currently taking ticagrelor and clinicians performing the invasive procedure should consult with the prescribing clinician before discontinuing such therapy.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1546-7
Bradyarrhythmias, including ventricular pauses, have occurred in patients receiving ticagrelor. In the The Study of Platelet Inhibition and Patient Outcomes (PLATO) study, Holter monitor-detected ventricular pauses of at least 3 seconds were reported more frequently during the first week of therapy in patients receiving ticagrelor than in those receiving clopidogrel (5.8 versus 3.6%, respectively). There was no difference in the overall risk of clinically important bradycardic effects (e.g., syncope, need for pacemaker insertion) between the treatment groups. Ventricular pauses were mostly asymptomatic and attributed to sinoatrial nodal suppression. Patients with a baseline increased risk of bradycardia (e.g., those with sick sinus syndrome, second- or third-degree AV block, syncope due to bradycardia without a pacemaker) were excluded from the PLATO study; therefore, some clinicians recommend that ticagrelor be used with caution in such patients.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1547
For more Drug Warnings (Complete) data for Ticagrelor (15 total), please visit the HSDB record page.
Ticagrelor is indicated to reduce the risk of cardiovascular death, myocardial infarction, and stroke in patients with acute coronary syndrome or a history of myocardial infarction. Ticagrelor is also indicated to reduce the risk of a first myocardial infarction or stroke in high risk patients with coronary artery disease.
FDA Label
Brilique, co administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with
- acute coronary syndromes (ACS) or
- a history of myocardial infarction (MI) and a high risk of developing an atherothrombotic event
Brilique, co-administered with acetyl salicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with a history of myocardial infarction (MI occurred at least one year ago) and a high risk of developing an atherothrombotic event.
Possia, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndromes (unstable angina, non-ST-elevation myocardial infarction [NSTEMI] or ST-elevation myocardial infarction [STEMI]); including patients managed medically, and those who are managed with percutaneous coronary intervention (PCI) or coronary artery by-pass grafting (CABG).
Prevention of thromboembolic events
Ticagrelor is a P2Y12 receptor antagonist that inhibits the formation of thromboses to reduce the risk of myocardial infarction and ischemic stroke. It has a moderate duration of action as it is given twice daily, and a wide therapeutic index as high single doses are well tolerated. Patients should be counselled regarding the risk of bleeding, dyspnea, and bradyarrhythmias.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Purinergic P2Y Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P2Y RECEPTORS. Included under this heading are antagonists for specific P2Y receptor subtypes. (See all compounds classified as Purinergic P2Y Receptor Antagonists.)
B01AC24
B01AC24
B01AC24
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC24 - Ticagrelor
Absorption
Ticagrelor is 36% orally bioavailable. A single 200mg oral dose of ticagrelor reaches a Cmax of 923ng/mL, with a Tmax of 1.5 hours and an AUC of 6675ng\*h/mL. The active metabolite of ticagrelor reaches a Cmax of 264ng/mL, with a Tmax of 3.0 hours and an AUC of 2538ng\*h/mL.
Route of Elimination
A radiolabelled dose of ticagrelor is 57.8% recovered in feces and 26.5% recovered in urine. Less than 1% of the dose is recovered as the unmetabolized parent drug. The active metabolite AC-C124910XX makes up 21.7% of the recovery in the feces. The metabolite AR-C133913XX makes up 9.2% of the recovery in the urine and 2.7% of the recovery in the feces. Other minor metabolites are predominantly recovered in the urine.
Volume of Distribution
The steady state volume of distribution of ticagrelor is 88 L.
Clearance
The renal clearance of ticagrelor is 0.00584L/h.
The drug is metabolized principally by cytochrome P-450 (CYP) isoenzyme 3A4 to an active metabolite that has similar antiplatelet activity as the parent drug.Plasma concentrations of ticagrelor and its active metabolite increase in a dose-dependent manner with peak concentrations achieved within approximately 1.5 and 2.5 hours, respectively. Ticagrelor is primarily eliminated in the feces and to a lesser extent in urine; less than 1% of a dose is recovered in urine as the parent drug and active metabolite. ... Both ticagrelor and its active metabolite are extensively (more than 99%) bound to human plasma proteins. Administration with a high-fat meal increases systemic exposure of ticagrelor by 21% and decreases peak plasma concentrations of the active metabolite by 22%, but has no effect on peak plasma concentrations of ticagrelor or on systemic exposure to the active metabolite.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1548
Ticagrelor is rapidly absorbed following oral administration.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1548
The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion.
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 27, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
/MILK/ It is not known whether ticagrelor or its active metabolites are excreted in human milk. Ticagrelor is excreted in rat milk.
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 27, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
For more Absorption, Distribution and Excretion (Complete) data for Ticagrelor (6 total), please visit the HSDB record page.
The complete structure of all ticagrelor metabolites are not well defined. Ticagrelor can be dealkylated at postition 5 of the cyclopentane ring to form the active AR-C124910XX. AR-C124910XX's cyclopentane ring can be further glucuronidated or the alkyl chain attached to the sulfur can be hydroxylated. Ticagrelor can also be glucuronidated or hydroxylated. Ticagrelor can also be N-dealkylated to form AR-C133913XX, which is further glucuronidated or hydroxylated.
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30-40% of the exposure of ticagrelor.
NIH; DailyMed. Current Medication Information for Brilinta (Ticagrelor) Tablet (Updated: September 2015). Available from, as of January 27, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7b3f443-e83d-4bf2-0e96-023448fed9a8
The drug is metabolized principally by cytochrome P-450 (CYP) isoenzyme 3A4 to an active metabolite that has similar antiplatelet activity as the parent drug.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1548
Ticagrelor is a reversibly binding oral P2Y(12) receptor antagonist in development for the prevention of thrombotic events in patients with acute coronary syndromes. The pharmacokinetics, metabolism, and excretion of ticagrelor were investigated over 168 hr in six healthy male subjects receiving a single oral suspension dose of 200 mg of (14)C-ticagrelor. ... Major circulating components in the plasma and feces were identified as ticagrelor and AR-C124910XX, whereas in urine the major components were metabolite M5 (AR-C133913XX) and its glucuronide conjugate M4. Levels of unchanged ticagrelor and AR-C124910XX were <0.05% in the urine, indicating that renal clearance of ticagrelor and AR-C124910XX is of minor importance. Interindividual variability was small in both urine and fecal extracts with only small quantitative differences. All 10 of the metabolites were fully or partially characterized and a full biotransformation pathway was proposed for ticagrelor, in which oxidative loss of the hydroxyethyl side chain from ticagrelor forms AR-C124910XX and a second oxidative pathway leads to N-dealkylation of ticagrelor, forming AR-C133913XX.
PMID:20551239 Teng R et al; Drug Metab Dispos 38 (9): 1514-21 (2010)
Ticagrelor has a plasma half life of approximately 8 hours, while the active metabolite has a plasma half life of approximately 12 hours.
The mean terminal half-lives of ticagrelor and its active metabolite reportedly are about 7 and 9 hours, respectively.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1548
Ticagrelor is a reversibly binding oral P2Y(12) receptor antagonist in development for the prevention of thrombotic events in patients with acute coronary syndromes. The pharmacokinetics, metabolism, and excretion of ticagrelor were investigated over 168 hr in six healthy male subjects receiving a single oral suspension dose of 200 mg of (14)C-ticagrelor. In most subjects, radioactivity was undetectable in plasma after 20 hr and whole blood after 12 hr (half-life values of 6.3 and 4.6 hr, respectively).
PMID:20551239 Teng R et al; Drug Metab Dispos 38 (9): 1514-21 (2010)
Ticagrelor is a P2Y12 receptor antagonist. The P2Y12 receptor couples with Gi2 and other Gi proteins which inhibit adenylyl cyclase. Gi mediated signalling also activates PI3K, Akt, Rap1b, and potassium channels. The downstream effects of these activities mediate hemostasis and lead to platelet aggregation. Antagonism of the P2Y12 receptor reduces development of occlusive thromboses, which can reduce the risk of myocardial infarction and ischemic stroke.
Ticagrelor, a cyclopentyltriazolopyrimidine derivative, is a nonthienopyridine, P2Y12 platelet adenosine diphosphate (ADP)-receptor antagonist. In contrast to the thienopyridines (e.g., clopidogrel, prasugrel), ticagrelor binds reversibly to the P2Y12 ADP receptor and does not require hepatic transformation to exert its pharmacologic effect. Ticagrelor exhibits noncompetitive and reversible binding to the P2Y12 platelet ADP receptor, preventing signal transduction of the cyclic adenosine monophosphate (cAMP) pathway. This results in reduced exposure of fibrinogen binding sites to the platelet glycoprotein (GP) IIb/IIIa complex and subsequent inhibition of platelet activation and aggregation. In addition to platelet ADP-receptor blockade, ticagrelor inhibits reuptake of adenosine into erythrocytes, a mechanism that may account for some of the beneficial cardiovascular effects of the drug, while potentially contributing to some of its adverse effects (e.g., dyspnea, ventricular pauses).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1547