

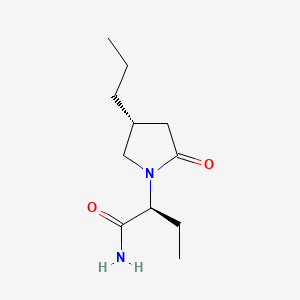

1. (2s)-2-((4r)-2-oxo-4-propylpyrrolidin-1-yl)butanamide
2. 1-pyrrolidineacetamide, Alpha-ethyl-2-oxo-4-propyl-, (alphas,4r)-
3. 2-(2-oxo-4-propylpyrrolidin-1-yl)butanamide
4. Briviact
5. Ucb 34714
6. Ucb-34714
7. Ucb34714
1. 357336-20-0
2. Briviact
3. Ucb-34714
4. Ucb 34714
5. Ucb34714
6. 2-(2-oxo-4-propylpyrrolidin-1-yl)butanamide
7. (2s)-2-[(4r)-2-oxo-4-propylpyrrolidin-1-yl]butanamide
8. (2s)-2-((4r)-2-oxo-4-propylpyrrolidin-1-yl)butanamide
9. U863jgg2ia
10. Rikelta
11. Brivaracetam [usan:inn]
12. Unii-u863jgg2ia
13. Compound 83alpha
14. 1-pyrrolidineacetamide, Alpha-ethyl-2-oxo-4-propyl-, (alphas,4r)-
15. Brivaracetam 97%
16. Briviact (tn)
17. Brivaracetam [mi]
18. Brivaracetam [inn]
19. Brivaracetam [jan]
20. Brivaracetam [usan]
21. Brivaracetam [mart.]
22. Brivaracetam [who-dd]
23. Schembl122081
24. Brivaracetam (jan/usan/inn)
25. Chembl607400
26. Gtpl9041
27. Dtxsid00905081
28. Chebi:133013
29. Brivaracetam [orange Book]
30. Ex-a2748
31. Zinc3979899
32. Bdbm50422531
33. Mfcd25976668
34. Akos027324306
35. Ccg-266666
36. Cs-3418
37. Db05541
38. Ncgc00390779-02
39. Ac-29289
40. As-35277
41. Bb161996
42. Hy-14449
43. D08879
44. Q408099
45. (s)-2-((r)-2-oxo-4-propylpyrrolidin-1-yl)butanamide
46. (2s)-2-((4r)-2-oxo-4-n-propyl-1-pyrrolidinyl)butanamide
47. (2s)-2-((4r)-2-oxo-4-propyltetrahydro-1h-pyrrol-1-yl) Butanamide
48. 1-pyrrolidineacetamide, .alpha.-ethyl-2-oxo-4-propyl (.alpha.s,4r)-
| Molecular Weight | 212.29 g/mol |
|---|---|
| Molecular Formula | C11H20N2O2 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 212.152477885 g/mol |
| Monoisotopic Mass | 212.152477885 g/mol |
| Topological Polar Surface Area | 63.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used as adjunctive therapy for partial-onset seizures in patients 16 years of age or older.
FDA Label
Briviact is indicated as adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalisation in adult and adolescent patients from 16 years of age with epilepsy.
Treatment of epilepsy with partial-onset seizures, Treatment of neonatal seizures
Treatment of neonatal seizures, Treatment of paediatric epilepsy syndromes
Treatment of epilepsy with partial-onset seizures
Brivaracetam binds SV2A with high affinity. SV2A is known to play a role in epileptogenesis through modulation of synaptic GABA release. It is thought that brivaracetam exerts its anti-epileptogenic effects through its binding to SV2A. Brivaracetam is also known to inhibit Na+ channels which may also contribute to its anti-epileptogenic action.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX23
N03AX23
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX23 - Brivaracetam
Absorption
Nearly 100% oral bioavailability.
Route of Elimination
\>95% excreted in urine with <10% of the parent compound unchanged. <1% excreted in feces.
Volume of Distribution
0.5L/kg.
Clearance
CL/F of 0.7-1.07 mL/min kg. Clearance is primarily metabolic with less than 10% of the parent drug excreted unchanged.
Primarily metabolized by hydrolysis of the acetamide moeity to form a carboxylic acid metabolite. Another metabolite is created via oxidation of the propyl side chain by CYP2C8 as well as CYP3A4, CYP2C19, and CYP2B6. Some conjugation with glucuronic acid and taurine account for a small amount of metabolism.
7-8h.
The precise mechanism of brivaracetam's anti-epileptogenic activity is unknown.