


1. Ambodryl
2. Bromodiphenhydramine
3. Bromodiphenhydramine Hydrochloride
1. Bromodiphenhydramine
2. Bromdiphenhydramine
3. 118-23-0
4. Histabromamine
5. Deserol
6. Neo-benadryl
7. Bromazina
8. Bromazinum
9. Amodryl
10. Ambodryl
11. Bromazin
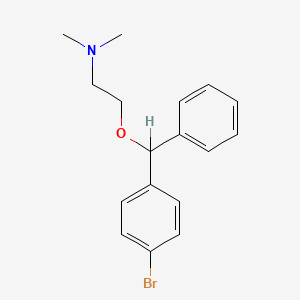
12. 2-[(4-bromophenyl)-phenylmethoxy]-n,n-dimethylethanamine
13. 2-(p-bromo-alpha-phenylbenzyloxy)-n,n-dimethylethylamine
14. Beta-(p-bromobenzhydryloxy)ethyldimethylamine
15. 2-((4-bromophenyl)(phenyl)methoxy)-n,n-dimethylethanamine
16. N-2-(4-bromobenzhydryloxy)ethyldimethylamine
17. Beta-dimethylaminoethyl P-bromobenzhydryl Ether
18. Chebi:59177
19. Bromdiphenhydraminum
20. T032bi7727
21. 2-[(4-bromophenyl)(phenyl)methoxy]-n,n-dimethylethanamine
22. Ethanamine, 2-[(4-bromophenyl)phenylmethoxy]-n,n-dimethyl-
23. Bromo-benadryl
24. Bromazine [inn:ban]
25. 2-(4-bromobenzhydryloxy)-nn-dimethylethylamine
26. Bromazinum [inn-latin]
27. Bromazina [inn-spanish]
28. Ethanamine, 2-((4-bromophenyl)phenylmethoxy)-n,n-dimethyl-
29. (6r,7r)-7-[[(z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,dihydrate
30. Einecs 204-238-8
31. Bromazin Base
32. Deserol Base
33. Unii-t032bi7727
34. Bromo-benadryl Base
35. Bromodiphen-hydramine
36. 4-bromodiphen Hydramine
37. Bromazine [inn]
38. Diphenhydramine Impurity C
39. Dsstox_cid_2688
40. Bromazine [who-dd]
41. Dsstox_rid_97539
42. Dsstox_gsid_22688
43. Schembl29806
44. Gtpl7132
45. Dimenhydrinate Impurity H
46. Chembl1201245
47. Dtxsid6022688
48. Bdbm81465
49. Bromodiphenhydramine [mi]
50. (+/-)-bromodiphenhydramine
51. 2-{[(4-bromophenyl)(phenyl)methyl]oxy}-n,n-dimethylethanamine
52. Bromodiphenhydramine [vandf]
53. Cas_2444
54. Hy-b1568
55. Nsc_2444
56. Tox21_113667
57. Pdsp1_000145
58. Pdsp2_000144
59. Bromodiphenhydramine, (+/-)-
60. Db01237
61. Ncgc00249891-01
62. Cas-118-23-0
63. Cs-0013459
64. .beta.-(p-bromobenzhydryloxy)ethyldimethylamine
65. 4-bromodiphen Hydramine [usp Impurity]
66. Dimenhydrinate Impurity H [ep Impurity]
67. L001164
68. Sr-01000944388
69. Q4926102
70. Sr-01000944388-1
71. {2-[(4-bromophenyl)(phenyl)methoxy]ethyl}dimethylamine
72. 2-[(4-bromophenyl)(phenyl)methoxy]-n,n-dimethylethanamine #
73. 2-[(p-bromo-.alpha.-phenylbenzyl)oxy]-n,n-dimethylethylamine
74. Diphenhydramine Hydrochloride Impurity C [ep Impurity]
75. Ethylamine, 2-[(p-bromo-.alpha.-phenylbenzyl)oxy]-n,n-dimethyl-
| Molecular Weight | 334.2 g/mol |
|---|---|
| Molecular Formula | C17H20BrNO |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 333.07283 g/mol |
| Monoisotopic Mass | 333.07283 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 258 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For management of symptoms related to hay fever and other types of allergy and used to help bring up phlegm, thin secretions, and make a cough productive.
Bromodiphenhydramine is an antihistamine of the ethanolamine class. Ethanolamine antihistamines have significant antimuscarinic activity and produce marked sedation in most patients. In addition to the usual allergic symptoms, the drug also treats irritant cough and nausea, vomiting, and vertigo associated with motion sickness. It also is used commonly to treat drug-induced extrapyramidal symptoms as well as to treat mild cases of Parkinson's disease. Rather than preventing the release of histamine, as do cromolyn and nedocromil, Bromodiphenhydramine competes with free histamine for binding at HA-receptor sites. Bromodiphenhydramine competitively antagonizes the effects of histamine on HA-receptors in the GI tract, uterus, large blood vessels, and bronchial muscle. Ethanolamine derivatives have greater anticholinergic activity than do other antihistamines, which probably accounts for the antidyskinetic action of Bromodiphenhydramine. This anticholinergic action appears to be due to a central antimuscarinic effect, which also may be responsible for its antiemetic effects, although the exact mechanism is unknown.
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AA - Aminoalkyl ethers
R06AA01 - Bromazine
Absorption
Well absorbed in the digestive tract.
Hepatic (cytochrome P-450 system); some renal.
1 to 4 hours
Bromodiphenhydramine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding.