


1. Bronchospasmin
2. Epiferol
3. Reproterol
4. Reproterol Monohydrochloride
5. Reproterol, (-)-isomer
1. Bronchodil
2. Bronchospasmin
3. 13055-82-8
4. Asmaterolo
5. Reproterol Hydrochloride [usan]
6. W-2946m
7. D-1959.hcl
8. Y4i1coj8w8
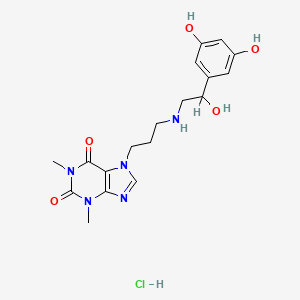
9. 7-[3-[[2-(3,5-dihydroxyphenyl)-2-hydroxyethyl]amino]propyl]-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione Monohydrochloride
10. 13055-82-8 (hcl)
11. 1h-purine-2,6-dione, 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-3,7-dihydro-1,3-dimethyl-, Monohydrochloride
12. Reproterol Hydrochloride (usan)
13. Reproterol Hcl
14. 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione Hydrochloride
15. 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione Monohydrochloride
16. 7-[3-[[2-(3,5-dihydroxyphenyl)-2-hydroxyethyl]amino]propyl]-1,3-dimethylpurine-2,6-dione;hydrochloride
17. D 1959 Hcl
18. Einecs 235-942-3
19. Unii-y4i1coj8w8
20. Einecs 254-666-4
21. Reproterolhydrochlorid
22. D 1959 (-)
23. 7-(3-((beta,3,5-trihydroxyphenethyl)amino)propyl)theophylline Monohydrochloride
24. 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxy-ethyl)amino)propyl)theophylline Hydrochloride
25. 1h-purine-2,6-dione, 3,7-dihydro-7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-1,3-dimethyl-, Monohydrochloride, (-)-
26. 39878-37-0
27. Schembl637198
28. D-1959 Hydrochloride
29. Chembl2104771
30. Dtxsid60926797
31. Reproterol Hydrochloride [mi]
32. Akos022180842
33. Reproterol Hydrochloride [mart.]
34. Theophylline, 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-, Hydrochloride
35. Reproterol Hydrochloride [who-dd]
36. 62932-28-9
37. 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione Hydrochloride
38. D05718
39. Q27294256
40. 7-(3-((.beta.,3,5-trihydroxyphenethyl)amino)propyl)theophylline Monohydrochloride
41. Theophylline, 7-(3-((.beta.,3,5-trihydroxyphenethyl)amino)propyl)-, Hydrochloride
42. 1h-purine-2,6-dione, 7-(3-((2-(3,5-dihydroxyphenyl)-2-hydroxyethyl)amino)propyl)-3,7-dihydro-1,3-dimethyl-, Hydrochloride (1:1)
43. 7-(3-{[2-(3,5-dihydroxyphenyl)-2-hydroxyethyl]amino}propyl)-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione--hydrogen Chloride (1/1)
44. 7-[3-[[2-(3,5-dihydroxyphenyl)-2-hydroxyethyl]amino]propyl]-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-d
| Molecular Weight | 425.9 g/mol |
|---|---|
| Molecular Formula | C18H24ClN5O5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 425.1465966 g/mol |
| Monoisotopic Mass | 425.1465966 g/mol |
| Topological Polar Surface Area | 131 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 569 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Adrenergic beta-Agonists
Drugs that selectively bind to and activate beta-adrenergic receptors. (See all compounds classified as Adrenergic beta-Agonists.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)