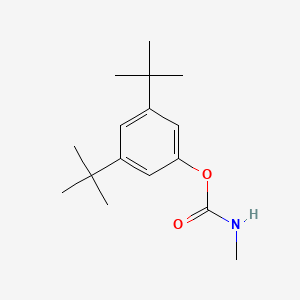

1. Butacarb [iso]
2. 2655-19-8
3. 3,5-di-tert-butylphenyl Methylcarbamate
4. 48sle5y118
5. Butacarbe [french]
6. Caswell No. 291b
7. Butacarb [iso:bsi]
8. Butacarbe
9. Hsdb 2799
10. 3,5-di-t-butylphenylmethylcarbamate
11. Bts 14639
12. Epa Pesticide Chemical Code 291300
13. Brn 1914596
14. Rd 14639
15. 3,5-di-tert-butylphenyl N-methylcarbamate
16. Unii-48sle5y118
17. 3,5-bis(1,1-dimethylethyl)phenol Methylcarbamate
18. Carbamic Acid, Methyl-, 3,5-di-tert-butylphenyl Ester
19. Phenol, 3,5-bis(1,1-dimethylethyl)-, Methylcarbamate
20. Butacarb [hsdb]
21. Schembl77964
22. Dtxsid0041690
23. Chebi:82089
24. C18949
25. Q27155741
26. 3,5-bis(1,1-dimethylethyl)phenyl N-methylcarbamate
| Molecular Weight | 263.37 g/mol |
|---|---|
| Molecular Formula | C16H25NO2 |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 263.188529040 g/mol |
| Monoisotopic Mass | 263.188529040 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 290 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
3. 3= moderately toxic: probable oral lethal dose (human) 0.5-5 g/kg, between 1 oz & 1 pint (or 1 lb) for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-305
In vivo studies have shown that carbamates are almost completely absorbed during normal transit through the gastrointestinal tract. ...In mice, all carbamates were rapidly distributed to the tissues and organs. The half-life values of penetration ranged from 8-17 min for carbamates. ...In animals, oxidation of carbamates often, but not always, results in detoxification. Oxidative metabolism generally leads to products of greater polarity and water solubility, and these can be more readily eliminated through the urine and feces than the parent compound. /Carbamate pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of December 28, 2004: https://www.inchem.org/documents/ehc/ehc/ehc64.htm
Little information is available on the distribution of carbamates in the various organs and tissues in mammals following exposure by inhalation or the oral route. The organs in which residues have been reported are the liver, kidneys, brain, fat, and muscle. The half-life in the rat is of the order of 3-8 hr. It seems that the excretion of carbamates via urine is also rapid in man, and that the metabolic pathways in man are the same as those in the rat. /Carbamate pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of July 3,2003: https://www.inchem.org/pages/ehc.html
When butacarb was incubated with mouse liver 10,000xg supernatant, eleven metabolites were detected. Hydrolysis produced only 6 phenols. One of these, the major metabolite, was identified as 3,5-di-tert-butylphenol. Other metabolites were characterized but not all were completely identified: two acid metabolites containing a carboxyl & carbamoyl group; two containing a carbamoyl & two hydroxy groups (but not dihydroxybenzenoid); a hydroxybutylphenol; one containing a carbamoyl & hydroxy group; two dihydroxybutylphenols; n-hydroxymethyl butacarb; & n-hydroxymethyl hydroxybutyl analog. Studies with housefly (musca domestica), blowfly (lucilia sericata) & grass grubs (costelytra zealandica) gave results quant similar to those with mouse liver enzymes.
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 66
...Butacarb.../is/ cleaved to phenolic products by enzyme system in liver microsomes, which requires nadph & is inhibited by carbon monoxide...it is likely that this...microsomal mono-oxygenation is very important in 'hydrolysis' of carbamates.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 378
The principal route of metabolism of insecticidal carbamate esters is oxidative and is generally associated with the mixed-function oxidase (MFO) enzymes, which are present in several tissues. /Carbamate pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of December 28, 2004: https://www.inchem.org/documents/ehc/ehc/ehc64.htm
The metabolism of 3,5-di-tert-butylphenyl-N-methylcarbamate (butacarb) was studied in insects and mouse liver enzymes. Tritium (H-3) labeled butacarb was incubated with 10,000 times the force of gravity supernatants from mouse liver or housefly and blowfly enzyme preparations, obtained from their abdomens. The reaction mixtures were analyzed for metabolites. Eleven oxidation products were detected in each species. In-vivo, adult houseflies and blowflies were dosed topically with H-3 labeled butacarb. The insects were then extracted by grinding them with sand and ether. Ether extracts were examined for metabolites. The water soluble fraction was also examined. Both fractions consisted of complex mixtures. The ether fraction was hydrolyzed with sodium-hydroxide, and the water soluble portion was analyzed. Six phenolic metabolites were detected. The major phenolic product was 3,5-di-tert-butylphenol, which accounted for more than half of the dose.
PMID:5144737 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1178072 Douch PGC, Smith JN; Biochem J 125 (2): 395-400 (1971)
The carbamates alone weakly activated estrogen- or progesterone-responsive reporter genes in breast and endometrial cancer cells. All of the carbamates decreased estradiol- or progesterone-induced reporter gene activity in the breast and endometrial cancer cells. In whole cell competition binding assays, the carbamates demonstrated a limited capacity to displace radiolabeled estrogen or progesterone from /estrogen receptor/ or /progesterone receptor/. /Carbamates/
PMID:9126867 Klotz D et al; Life Sciences 60 (17): 1467-1475 (1997)
Carbamates are effective insecticides by virtue of their ability to inhibit acetylcholinesterase (AChE) in the nervous system. They also inhibit other esterases. The carbamylation of the enzyme is unstable, and the regeneration of AChE is relatively rapid compared with that from a phosphorylated enzyme. Thus, carbamate pesticides are less dangerous with regard to human exposure than organophosphorus pesticides. The ratio between the dose required to produce death and the dose required to produce minimum symptoms of poisoning is substantially larger for carbamate compounds than for organophosphorus compounds. /Carbamate Pesticides/
WHO; Environ Health Criteria 64: Carbamate pesticides (1986). Available from, as of July 3,2003: https://www.inchem.org/pages/ehc.html
The carbamate insecticides are reversible cholinesterase-inhibitors. They cause this effect by reversible carbamylation of the enzyme acetylcholinesterase, allowing accum of acetylcholine, as with the organophosphate insecticides. ... While the organophosphate insecticides cause irreversible inhibition of the cholinesterase enzymes, the carbamyl-enzyme complex is reversible & dissociates far more readily than the organophosphate complex. The clinical syndrome is more benign & of much shorter duration with the carbamate insecticides. /Carbamate insecticides/
Haddad, L.M. and Winchester, J.F. Clinical Management of Poisoning and Drug Overdosage. Philadelphia, PA: W.B. Saunders Co., 1983., p. 711